Medical device and MedTech insights, news, tips and more
FDA Grants Breakthrough Device Designation to Reflow Medical’s Temporary Spur Stent System
January 9, 2020
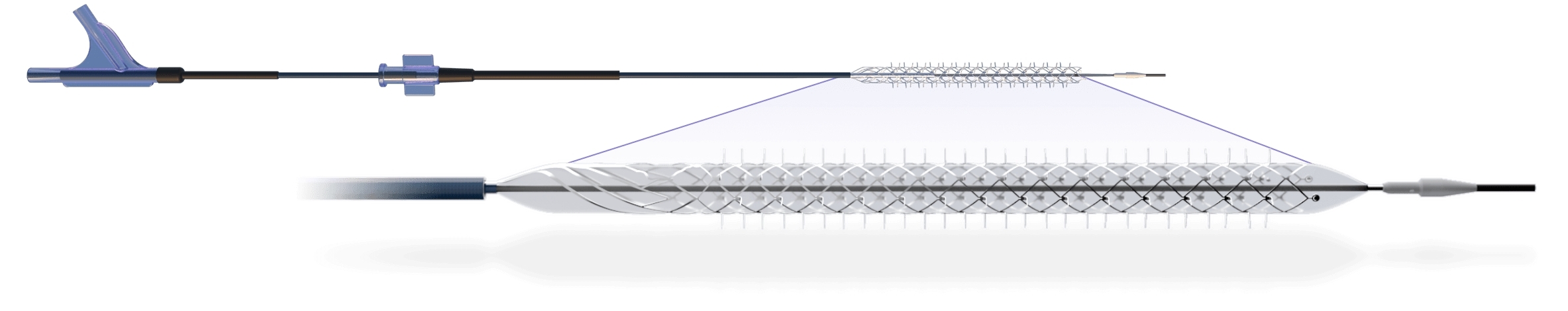
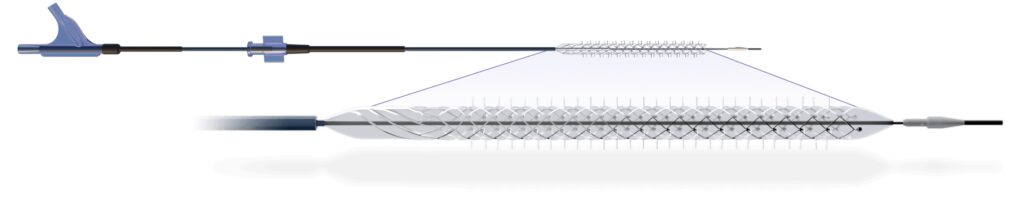
Reflow Medical announces that the Temporary Spur Stent System, a novel retrievable stent technology intended for the treatment of below-the-knee (BTK) peripheral artery disease, has been designated for the Breakthrough Devices Program by the U.S. Food and Drug Administration (FDA).
The Breakthrough Devices Program is designed to give patients and health care providers timely access to medical devices that provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions. The program offers Reflow Medical the opportunity to interact with experts at the FDA throughout the premarket review phase, in order to help speed the development, assessment and review of the device.
The Temporary Spur Stent System is a novel combination device consisting of a patented retrievable stent system having a series of radially expandable spikes designed to create multiple pathways to deliver antiproliferative drugs for increased uptake into the vessel wall and facilitate acute luminal gain, without leaving anything behind. The device was developed in response to unmet clinical needs resulting in high rates of restenosis and treatment challenges in patients with BTK disease.
“We are extremely grateful to the FDA for their expedited designation of the Temporary Spur Stent System as a Breakthrough Device. We plan to take full advantage of the Program’s benefits, accelerating our efforts towards meeting the requirements of the review process as we advance this novel technology, with the goal of improving the lives of patients,” says Isa Rizk, CEO of Reflow Medical.
As Reflow continues to build on clinical evidence supporting the Temporary Spur Stent System, the company looks forward to furthering development in other clinical areas, based on the Spur technology platform.
See Full Press Release: FDA Grants Breakthrough Device Designation to Reflow Medical’s Temporary Spur Stent System | Business Wire
Written by: Reflow Medical

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.