Medical device and MedTech insights, news, tips and more
First-of-its-Kind XACT Robotic System Cleared by FDA for Percutaneous Interventional Procedures
October 31, 2019

XACT Robotics™ Ltd. today announced that its first robotic system was cleared to market in the U.S. for use during computed tomography (CT) guided percutaneous interventional procedures. XACT’s technology is the first hands-free robotic system combining image-based planning and navigation with insertion and steering of various instruments to a desired target across an array of clinical applications and indications.
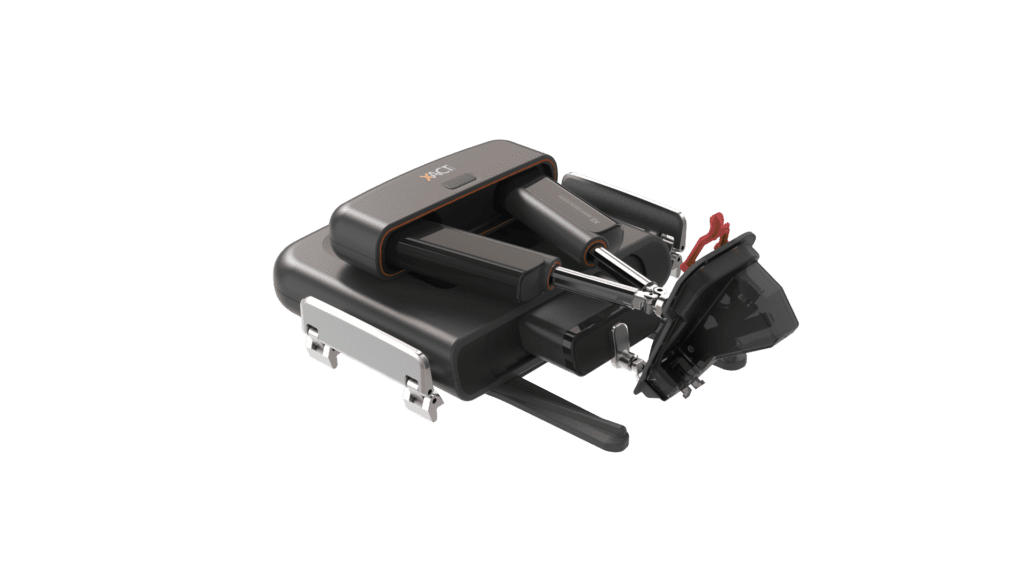
“We are committed to redefining the way the entire medical community utilizes robotics, beginning with interventional radiologists,” said Harel Gadot, Founder, Executive Chairman and President, XACT Robotics. “Being the first to introduce a hands-free robotic system, we have the potential to provide significant clinical, technical and economic value while democratizing interventional procedures. Our system’s small footprint and high mobility design will enable care providers to treat a broad range of patient care needs in various clinical sites of service.”
“The XACT Robotic System provides a unique platform to the interventional radiology community which can help improve the delivery and quality of care for the patients we serve,” said Professor Nahum S. Goldberg, M.D., who is the Principal Investigator in a multi-site study using the system, which is still an investigational device in Israel. Prof. Goldberg is the Head of Interventional Oncology Unit and Director of the Applied Radiology Research Lab at Hadassah Hebrew University Medical Center. “Based on our experience with this unique robotic technology, we can reach very small targets with unprecedented accuracy. Furthermore, this system holds much promise for enabling more efficient use of time and hospital resources.”
See Full Press Release: First-of-its-Kind XACT Robotic System Cleared by FDA for Percutaneous Interventional Procedures
Written by: XACT Robotics

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.
