Medical device and MedTech insights, news, tips and more
Fusion Robotics Wins FDA Clearance for 3D Imaging Robotic Targeting System
February 28, 2021
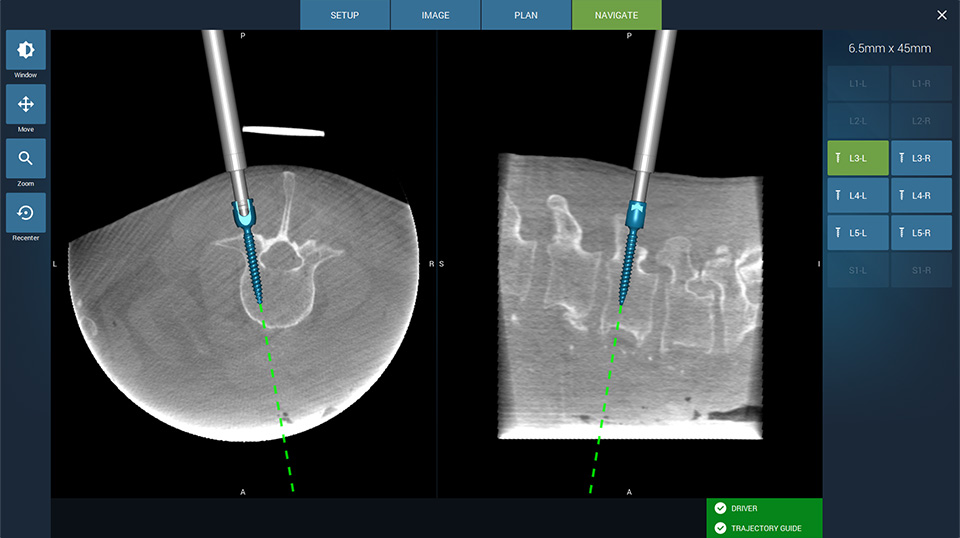
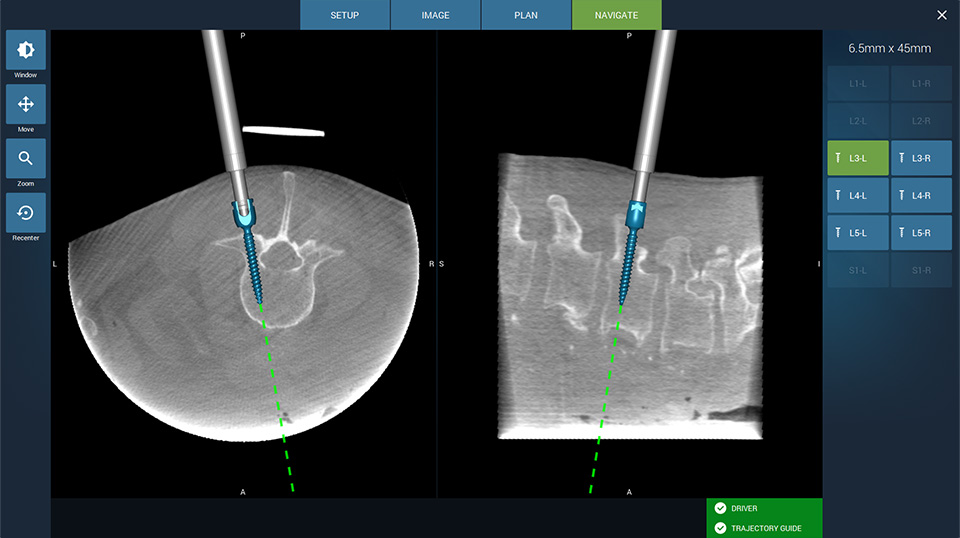
Fusion Robotics LLC, a spinal robotics and navigation company today announced receiving 510(k) clearance to market their 3D imaging compatible navigation and robotic targeting system for spine surgery in the U.S. market.
The Fusion Robotics System addresses the key limitations of current spinal navigation and robotics systems by offering greater procedural efficiency with significantly less expense.
“We appreciate the partnership developed with FDA to rigorously validate safety and accuracy. Next, we look forward to partnering with clinicians and hospitals to increase efficiency, reduce cost and broadly expand the application of robotics to treat patients.” Said Brad Clayton, CEO of Fusion Robotics.
Fusion Robotics Chief Medical Officer, Kevin Foley, MD commented that “Since the introduction of robotics in spine surgery, the questions of economics and efficiency have hindered the widespread adoption of this technology. With this clearance, and the rapid development of our fluoroscopy-integrated system, we’re working quickly towards making robotics available to many more surgeons, in a form better suited to their practices.”
The company plans to commercialize broadly across the U.S. in 2021.
About Fusion Robotics, LLC
Fusion Robotics, LLC (www.fusionroboticsusa.com) is a medical device manufacturer, headquartered in Boulder CO, which is focused on the research, development and commercialization of robotics technologies for spinal surgical applications. Working closely with partners Interventional Systems (www.interventional-systems.com) and Intellijoint Surgical (www.intellijointsurgical.com), the company’s focus is to provide pragmatic and economical navigation and robotics solutions for broad clinical use in spine surgery.
See Full Press Release at the Source: Fusion Robotics™ Receives 510(k) Clearance for Spinal Navigation & Robotics System
Press Release by: Fusion Robotics
 Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.