Medical device and MedTech insights, news, tips and more
HeartVista Receives FDA 510(k) Clearance for One Click™ Cardiac MRI Package, the First AI-assisted Cardiac MRI Scan Solution
October 29, 2019
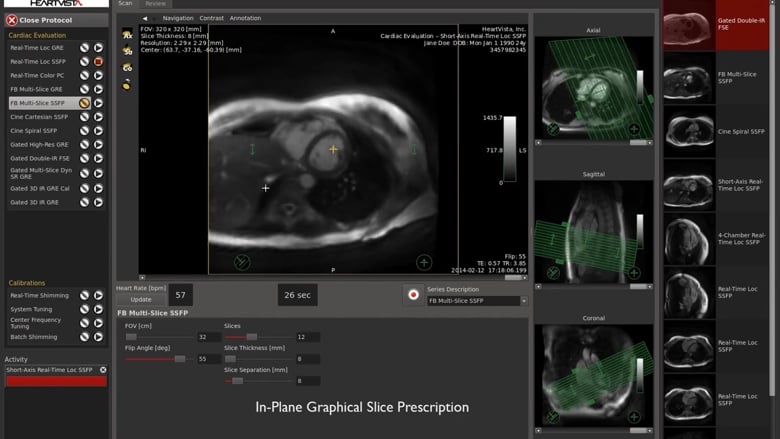
HeartVista, a pioneer in AI-assisted MRI solutions, today announced that it received 510(k) clearance from the U.S. Food and Drug Administration to deliver its AI-assisted One Click™ MRI acquisition software for cardiac exams. Despite the many advantages of cardiac MRI, or cardiac magnetic resonance (CMR), its use has been largely limited due to a lack of trained technologists, high costs, longer scan time, and complexity of use. With HeartVista’s solution, cardiac MRI is now simple, time-efficient, affordable, and highly consistent.
“HeartVista’s Cardiac Package is a vital tool to enhance the consistency and productivity of cardiac magnetic resonance studies, across all levels of CMR expertise,” said Dr. Raymond Kwong, MPH, Director of Cardiac Magnetic Resonance Imaging at Brigham and Women’s Hospital and Associate Professor of Medicine at Harvard Medical School.
A recent multi-center, outcome-based study (MR-INFORM), published in the New England Journal of Medicine, demonstrated that non-invasive myocardial perfusion cardiovascular MRI was as good as invasive FFR, the previous gold standard method, to guide treatment for patients with stable chest pain, while leading to 20% fewer catheterizations.
“This recent NEJM study further reinforces the clinical literature that cardiac MRI is the gold standard for cardiac diagnosis, even when compared against invasive alternatives,” said Itamar Kandel, CEO of HeartVista. “Our One Click™ solution makes these kinds of cardiac MRI exams practical for widespread adoption. Patients across the country now have access to the only AI-guided cardiac MRI exam, which will deliver continuous imaging via an automated process, minimize errors, and simplify scan operation. Our AI solution generates definitive, accurate and actionable real-time data for cardiologists. We believe it will elevate the standard of care for cardiac imaging, enhance patient experience and access, and improve patient outcomes.”
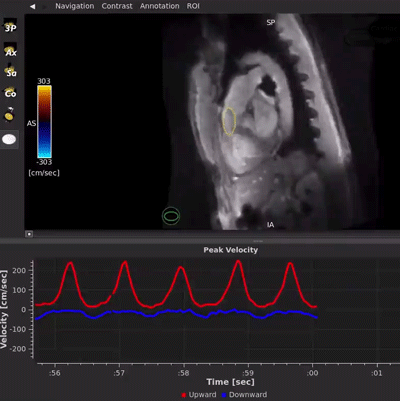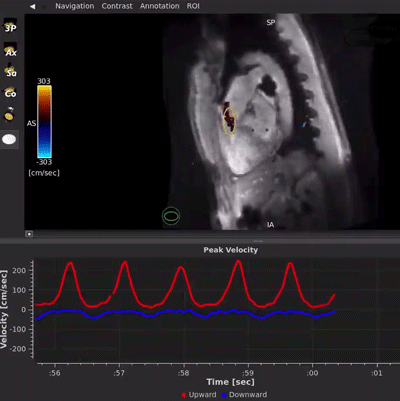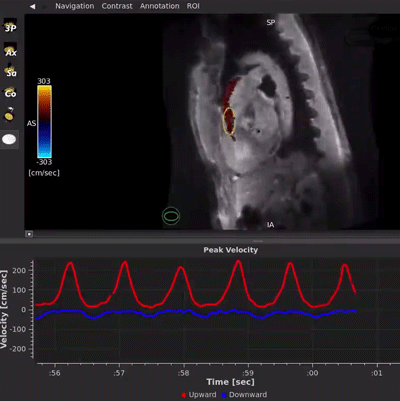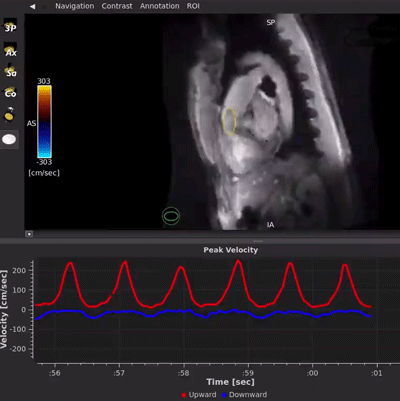
HeartVista’s FDA-cleared Cardiac Package uses AI-assisted software to prescribe the standard cardiac views with just one click, and in as few as 10 seconds, while the patient breathes freely. A unique artifact detection neural network is incorporated in HeartVista’s protocol to identify when the image quality is below the acceptable threshold, prompting the operator to reacquire the questioned images if desired. Inversion time is optimized with further AI assistance prior to the myocardial delayed-enhancement acquisition. A 4D flow measurement application uses a non-Cartesian, volumetric parallel imaging acquisition to generate high quality images in a fraction of the time. The Cardiac Package also provides preliminary measures of left ventricular function, including ejection fraction, left ventricular volumes, and mass.
See Full Press Release: HeartVista Receives FDA 510(k) Clearance for One Click™ Cardiac MRI Package, the First AI-assisted Cardiac MRI Scan Solution | Business Wire
Written by: Heartvista

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.