Medical device and MedTech insights, news, tips and more
IlluminOss Medical Receives FDA Clearance for Use in Femur and Tibia Fractures as a Supplement to Approved Hardware
October 29, 2020
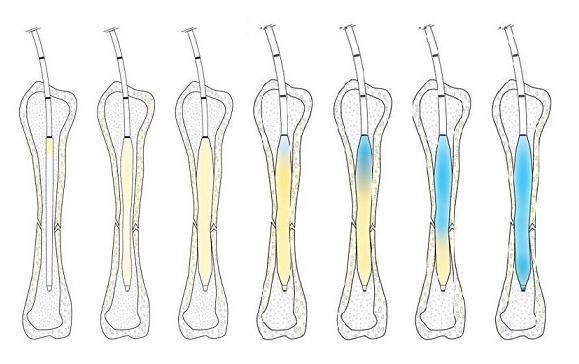
IlluminOss Medical, a medical device company focused on minimally invasive orthopedic fracture repair, today announced an expanded U.S. Food and Drug Administration (FDA 510k) clearance for its Photodynamic Bone Stabilization System. The new clearance allows IlluminOss to be used in femur and tibia fractures as supplemental fixation to FDA-cleared fracture fixation systems.
“Femur and tibia fractures can be challenging in aging patients where osteoporotic bone often lacks the strength required for reliable fixation with traditional hardware systems,” said Robert Rabiner, Chief Technology Officer of IlluminOss. “This new clearance allows the surgical community to use the IlluminOss intramedullary implant as a supplement to these systems and help prevent construct failures by dramatically improving the holding power of screws and other hardware.”
“Many lower limb fractures in the elderly, especially close to the knee joint, can be very difficult to achieve reliable stabilization due to poor bone quality,” said Mike Mogul, Chairman of IlluminOss. “As a minimally invasive means of supplementing hardware systems, IlluminOss provides substantial improvement in screw purchase and can optimize surgical outcomes. Surgeons can now securely affix their hardware anywhere along the length of the IlluminOss implant.”
The IlluminOss System is a minimally invasive approach for fracture repair and stabilization through a patient-conforming intramedullary implant. The system utilizes a light-curable liquid monomer, contained within an expandable balloon, to create a patient-conforming, rigid implant within the bone canal. The IlluminOss technology has been in clinical use in Europe since 2010, and in the US since 2018, with over 4,000 procedures to date.
In the US, the IlluminOss System is now indicated for use in skeletally mature patients in the treatment of traumatic, fragility, pathological, and impending pathological fractures of the humerus, radius, ulna, clavicle, pelvis, fibula, metacarpals, metatarsals, and phalanges. The IlluminOss Photodynamic Bone Stabilization System can also be used in conjunction with FDA-cleared fracture fixation systems to provide supplemental fixation in these anatomic sites. The IlluminOss System may be used in the femur and tibia to provide supplemental fixation to an anatomically appropriate FDA-cleared fracture fixation system.
See Full Press Release at the Source: IlluminOss Medical Receives FDA Clearance for Use in Femur and Tibia Fractures as a Supplement to Approved Hardware
Press Release by: IlluminOss Medical
 Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.


