Medical device and MedTech insights, news, tips and more
ImpediMed Receives FDA 510(k) Clearance of SOZO for Expanded Indication
December 12, 2019
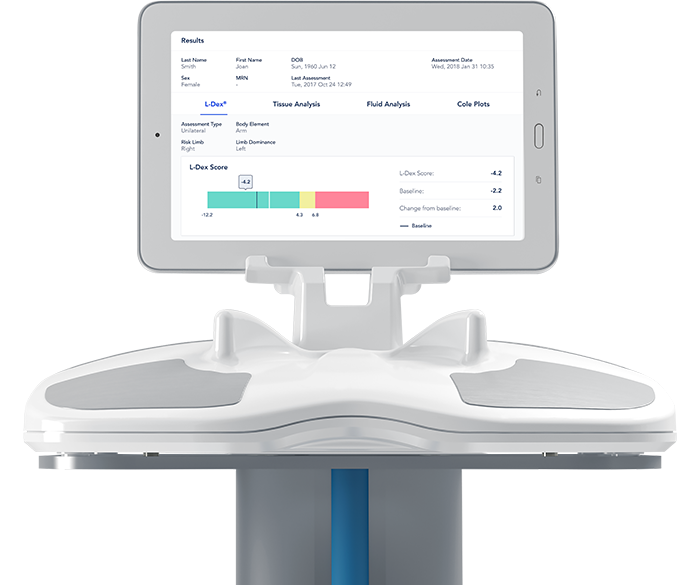
ImpediMed Limited (ASX.IPD), a medical software technology company that non-invasively measures, monitors and manages fluid status and tissue composition using bioimpedance spectroscopy (BIS), recently announced the issuance of a further 510(k) clearance for SOZO® by the U.S. Food and Drug Administration (FDA). SOZO is the first device to gain FDA clearance for use as an aid in the assessment of Protein Calorie Malnutrition.
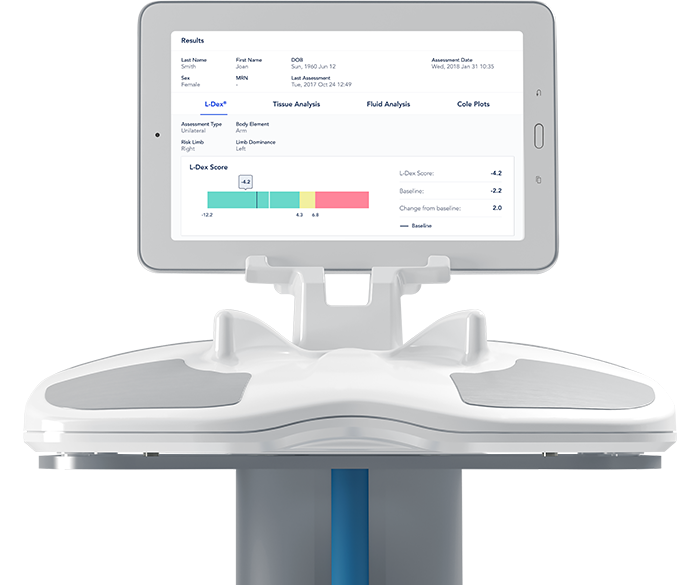
The expanded clearance enables ImpediMed to market SOZO for assessing patients at risk of Protein Calorie Malnutrition (PCM) and to track clinically relevant body composition parameters over time in both healthy and unhealthy patient populations. SOZO will aid clinicians who are using Subjective Global Assessment (SGA) tools to assess patients at risk of PCM.
Malnutrition is a largely under-recognized health problem. Greater than one-third of patients in the U.S. are malnourished prior to being admitted to the hospital. Further, one-third of patients not malnourished at the time of admission, become malnourished during their stay at the hospital.1
According to the U.S. National Cancer Institute, at the time of cancer diagnosis, 80% of patients with gastrointestinal cancer and 60% of patients with lung cancer have already experienced significant involuntary weight loss. Additionally, malnutrition is common among outpatients with heart failure (HF) and is strongly related to increased mortality. In a recent study to determine the prevalence and prognostic consequences of malnutrition in HF patients, they found 57% of the HF patients were at least mildly malnourished as measured by at least one nutritional index.2
Nationally, the annual direct medical cost of disease-associated malnutrition is over $15.5 billion.1
SGA tools such as the American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) guidelines define changes in physical attributes as assessment criteria for PCM in patients. SOZO provides quantitative values for fluid accumulation, muscle and fat mass to provide robust, objective data, critical to nutritional evaluations, and can be used by clinicians to support their assessment and diagnosis of PCM.
A recent study published in the Journal of the American Medical Association titled “Association of Nutritional Support With Clinical Outcomes Among Medical Inpatients Who Are Malnourished or at Nutritional Risk” demonstrates the clinical benefit of nutritional support in malnourished patients.
“Many chronic diseases, including cancer, COPD, CHF and liver disease, are associated with PCM,” according to Scott C. Howell, D.O., MPH & TM, Chief Medical Officer at Vitality HealthPlan. “The consequences can be significant, including increased mortality, prolonged hospital stays and poor wound healing. A proactive, screening-based approach for nutritional support, employing SOZO, could lead to significantly improved clinical outcomes for patients.”
See Full Press Release: ImpediMed Receives FDA 510(k) Clearance of SOZO® for Expanded Indication
Written by: ImpediMed

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.
