Medical device and MedTech insights, news, tips and more
Innovative Bedside Biologic Maker RedDress Receives 510(k) for RD1 System
July 16, 2018
RedDress Ltd announced today that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its RD1 system.
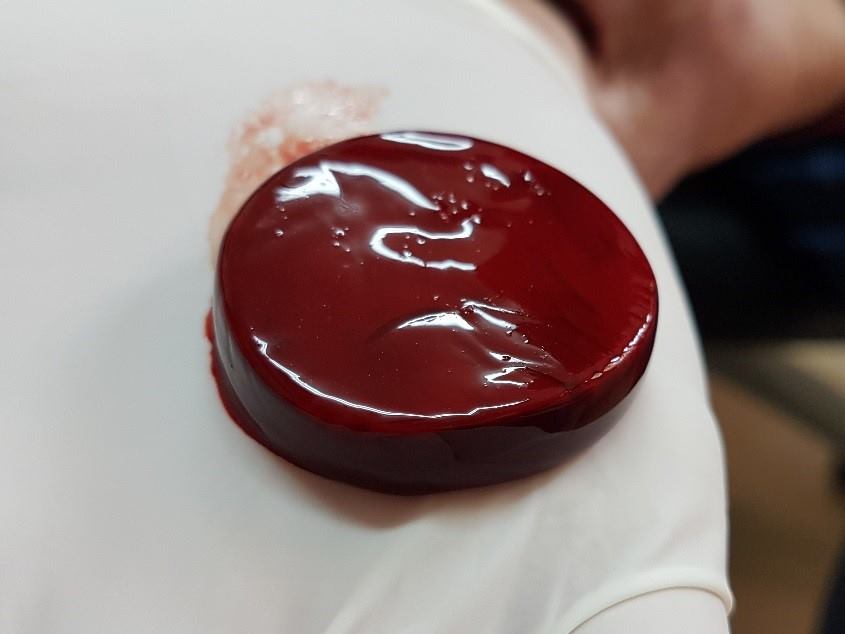
RD1 is an innovative wound care product which enables health care providers to produce in real time, in vitro whole blood clots, for use as a chronic wound care product. It has been developed to address the need for cost effectiveness and clinical effectiveness of wound care.
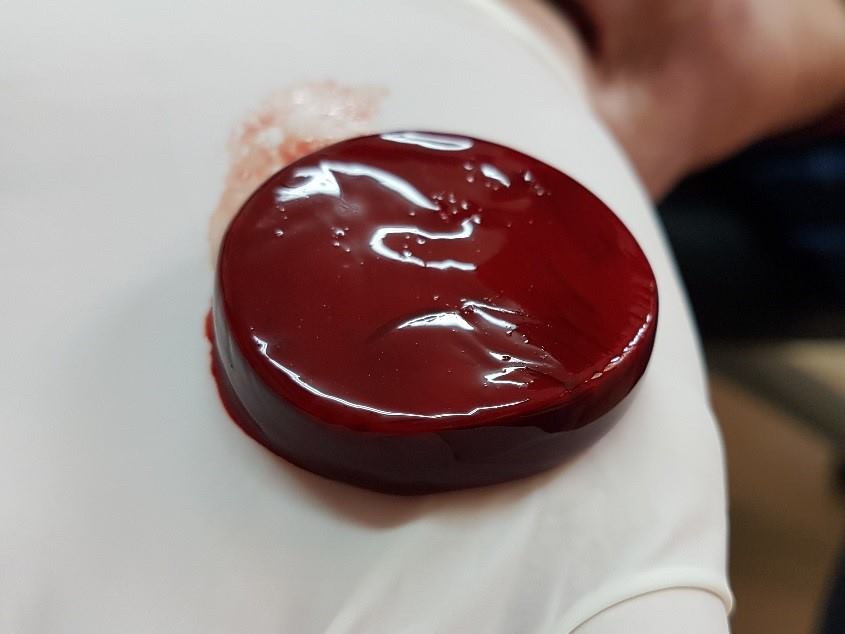 The RD1 System is intended to be used at point-of-care for the safe and rapid preparation of whole blood clot from a small sample of a patient’s own peripheral blood. Under the supervision of a healthcare professional, the whole blood clot produced by the RD1 System is topically applied for the management of exuding cutaneous wounds, such as leg ulcers, pressure ulcers, diabetic ulcers, and mechanically or surgically-debrided wounds.
The RD1 System is intended to be used at point-of-care for the safe and rapid preparation of whole blood clot from a small sample of a patient’s own peripheral blood. Under the supervision of a healthcare professional, the whole blood clot produced by the RD1 System is topically applied for the management of exuding cutaneous wounds, such as leg ulcers, pressure ulcers, diabetic ulcers, and mechanically or surgically-debrided wounds.
The 510(k) clearance allows RedDress to introduce its innovative device technology to the U.S. market, where it will be marketed as the RD1 System for the indications for use cited above.
“The recent FDA clearance is a significant milestone for RedDress,” said Alon Kushnir, Founder and CEO. “RedDress is pioneering a new technology for wound care management. RD1 is the first product utilizing our proprietary patented technology. Our mission is to provide wound care technology that is engineered to improve patient outcomes.”
RedDress, a private medical device company, developed a ground breaking, advanced wound care product, based on a patented technology to reproduce the natural process of the body to heal wounds. The company based in Israel, was founded in 2009 by Dr. Igal Kushnir, MD and Alon Kushnir and is focused on developing effective solutions that improve outcomes for patients. RedDress management has vast experience in bringing breakthrough medical ideas to successful commercialization. For more information, please visit www.reddress.co.il.
Full Press Release at the Source: RedDress Receives FDA 510(k) Clearance for RD1 System
Press Release by Redress Ltd. Images from Redress
 A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.

