Medical device and MedTech insights, news, tips and more
Intuitive Surgical Gains FDA Clearance for Lung Cancer Biopsy Robot
February 25, 2019
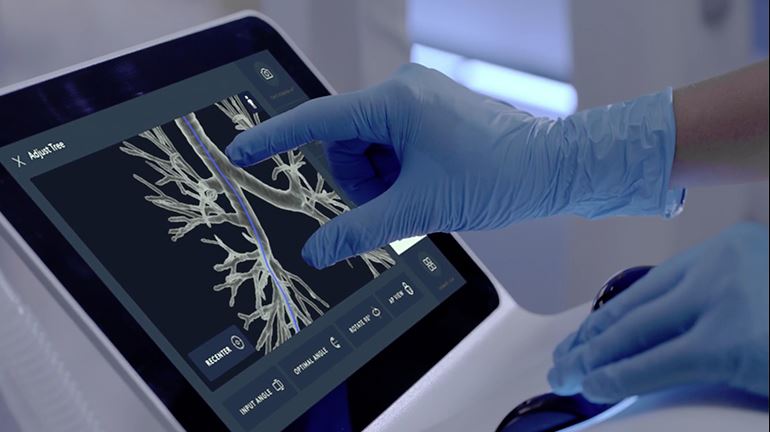
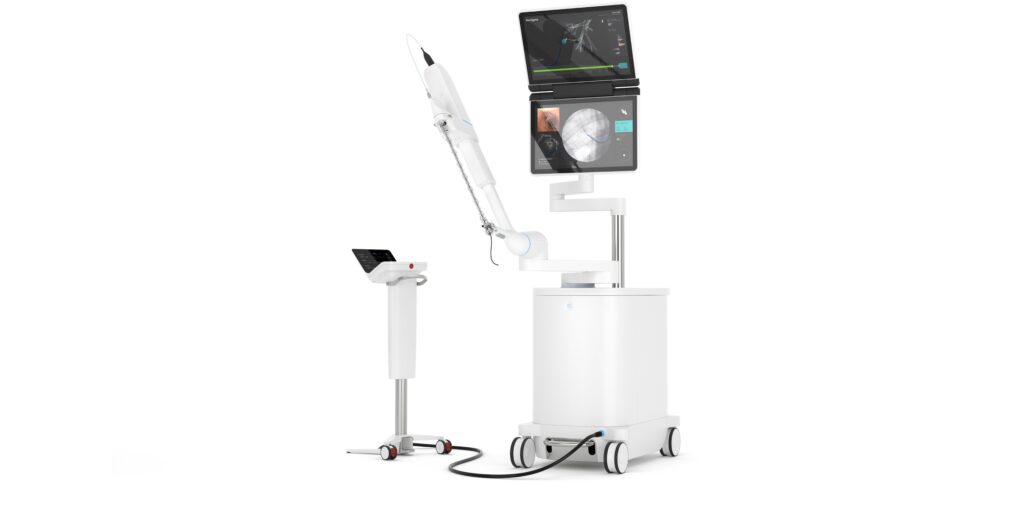
Intuitive Surgical Inc. said last week that the U.S. Food and Drug Administration has cleared its Ion system. The robotic endoluminal system is designed to enable doctors to conduct minimally invasive biopsies deep within the lung.
Ion includes an articulating robotic catheter with an outer diameter of only 3.5 mm that is able to move 180 degrees in all directions, allowing a physician to navigate the catheter through small and tortuous airways to reach nodules.
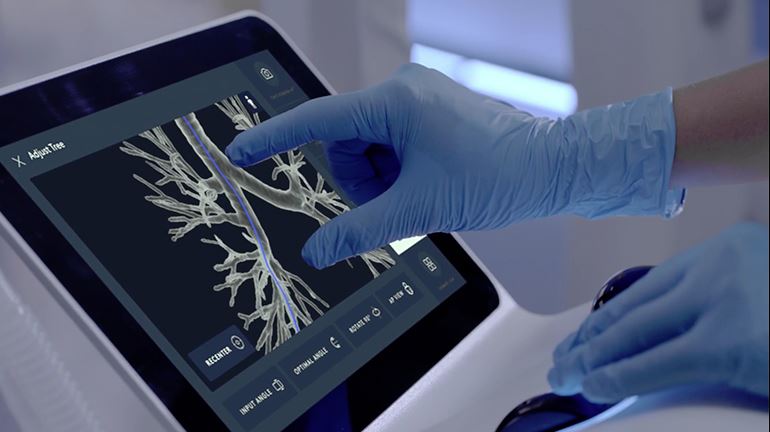
 Intuitive Surgical’s Flexision Biopsy Needle is also able to pass through tight bends as it travels through the Ion’s catheter to collect peripheral lung tissue. Other biopsy tools are also able to fit inside the catheter’s 2 mm working channel, including biopsy forceps or cytology brushes.
Intuitive Surgical’s Flexision Biopsy Needle is also able to pass through tight bends as it travels through the Ion’s catheter to collect peripheral lung tissue. Other biopsy tools are also able to fit inside the catheter’s 2 mm working channel, including biopsy forceps or cytology brushes.
Ion‘s fiber-optic, shape-sensor technology provides the catheter’s precise location and shape information throughout the procedure. Intuitive said it designed the Ion system to easily integrate into existing lung nodule biopsy workflows. Ion can also integrate with existing imaging technology, such as fluoroscopy, radial-endobronchial ultrasound, and cone-beam CT.
“The Ion system represents Intuitive’s continued commitment to innovating for minimally invasive care and extends our focus beyond surgery,” said Intuitive Surgical CEO Gary Guthart. “At Intuitive, we innovate for need, and lung cancer is clearly a global health challenge that requires new modalities of care.”
“Lung cancer is the world’s leading cause of cancer deaths,” stated Sunnyvale, Calif.-based Intuitive Surgical. “Many suspicious lesions found in the lung may be small and difficult to access, which can make obtaining a diagnosis challenging.”
Intuitive Surgical and its da Vinci system have been the market leaders for years. Physicians at Northwestern Memorial Hospital in Illinois recently used a da Vinci Xi for the first robot-assisted lung volume reduction surgery to treat severe emphysema resulting from chronic obstructive pulmonary disease.
However, Intuitive is facing increasing competition from companies such as Medtronic, Transenterix, and Verb Surgical. Johnson & Johnson last week acquired Auris Health, whose Monarch platform is intended for diagnostic and therapeutic bronchoscopic procedures. Corindus Vascular Robotics applied for FDA approval of its CorPath GRX system for neurological surgery.
“In the technology space, you have a lot people realizing the technologies necessary to build robots are more approachable than they have been in the last 10 years,” said Roger Smith, M.D., chief technology officer of the Florida Hospital Nicholson Center. “This means that a lot of the necessary pieces are now in place, and you can put a company together and put a device together that does something unique, and it won’t cost you $500 million to do it. That’s how you end up with 57 companies.”
See Full Article at the Source: Ion lung biopsy system from Intuitive Surgical wins FDA approval
Written by: Chris Newmarker
 A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.