Medical device and MedTech insights, news, tips and more
Itamar Medical Gets Favorable CMS Reimbursement Decision for WatchPAT Technology
November 9, 2018
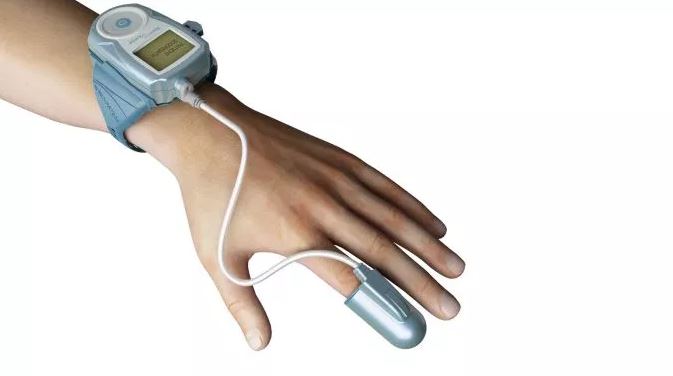
Itamar Medical Ltd. today announced the release of the 2019 Fee Schedule from the U.S. Centers for Medicare & Medicaid Services (CMS) that should support broad use of its WatchPAT technology.
WatchPAT is the only product on the market today that uses Peripheral Arterial Tone technology, providing cardiologists and sleep physicians in the United States with accurate sleep diagnosis that enhances clinical decision-making. The Company estimates that WatchPAT accounted for approximately 15% of the home sleep tests in the United States in 2017.
Gilad Glick, CEO of Itamar Medical said, “We believe that the new CMS Fee Schedule validates the clinical value of our sleep tests for patients and may significantly advance utilization of our innovative sleep tests in the U.S market. It also reflects growing awareness of deploying home sleep apnea testing and sleep apnea management as essential components of the cardiology care pathway.”
Read More at the Source: Itamar Medical’s WatchPAT™ Gets Favorable CMS Reimbursement Decision – RTSleepWorld
Press Release by Itamar Medical
 A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.