Medical device and MedTech insights, news, tips and more
Kerecis to Donate FDA-Approved Fish Skin for Burn Victims of Maui Wildfires
August 14, 2023
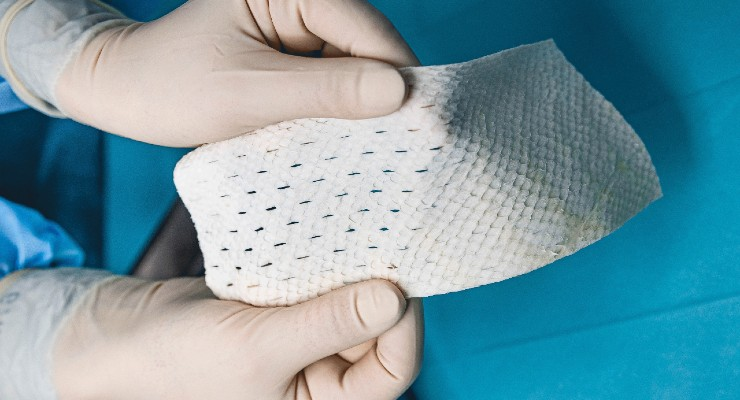
Kerecis®, the company pioneering the use of fish skin and fatty acids for tissue regeneration and protection, is donating its GraftGuide® fish-skin burn product for victims of the fires on Maui, Hawaii. Qualified medical personnel wanting to get fish-skin burn-treatment products for their patients should call 703-287-8752 or email wildfires@kerecis.com. Kerecis is dispatching a specialist to train doctors who would be using the product for the first time.
“Severe burns are life-threatening and extremely painful,” said Fertram Sigurjonsson, founder and CEO of Kerecis. “We want to help burn victims heal as quickly and fully as possible. We are contacting medical facilities in Maui and encourage medical professionals to contact us to get Kerecis GraftGuide fish skin for their patients.”
Kerecis had previously donated its burn products for victims of both the California wildfires in 2020 and the volcanic eruption on White Island in New Zealand in 2019.
About the Kerecis GraftGuide Fish Skin
The GraftGuide product line is intact fish skin that addresses the challenges of burn healing. The products are available in multiple sizes and forms. GraftGuide Standard is meshed fish skin, available in sheets of 250 cm2 (38 square inches) and 300 cm2 (46 square inches). GraftGuide Micro is intact fish skin divided into fragments, which mold into wound beds. And GraftGuide Mano is intact fish skin designed to fit the shape of the hand.
About Kerecis
Kerecis develops products from fish skin and fatty acids for cellular therapy, tissue regeneration and protection. When grafted onto damaged human tissue or implanted, the patented material supports the body’s own processes to heal and regenerate. Because no disease-transfer risk exists between cold-water fish and humans, the Kerecis fish skin is only gently processed and retains its similarity to human tissue. The gentle processing preserves the skin’s original three-dimensional structure, maintaining its inherent natural strength, complexity and molecules (such as fatty acids). Clinical studies have found that the Kerecis products heal wounds faster than competing products. Kerecis is the only approved manufacturer of medical devices containing intact fish skin globally.
See Full Press Release at the Source: Kerecis to Donate FDA-Approved Fish Skin for Burn Victims of Maui Wildfires
Press Release by: Kerecis
Legacy MedSearch has more than 35 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 17 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.

