Medical device and MedTech insights, news, tips and more
Limaca Medical Receives FDA Breakthrough Device Designation
May 13, 2022
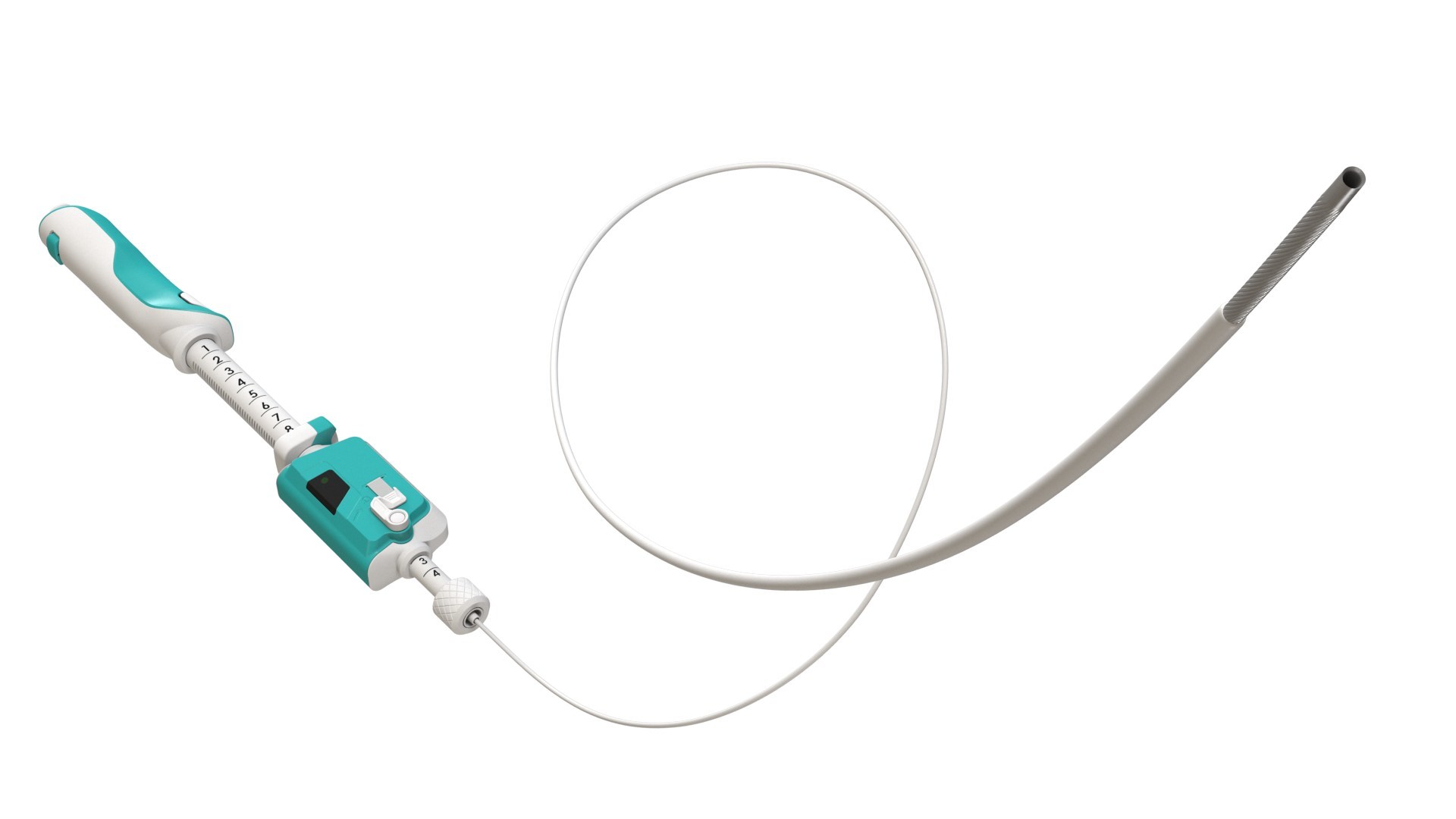
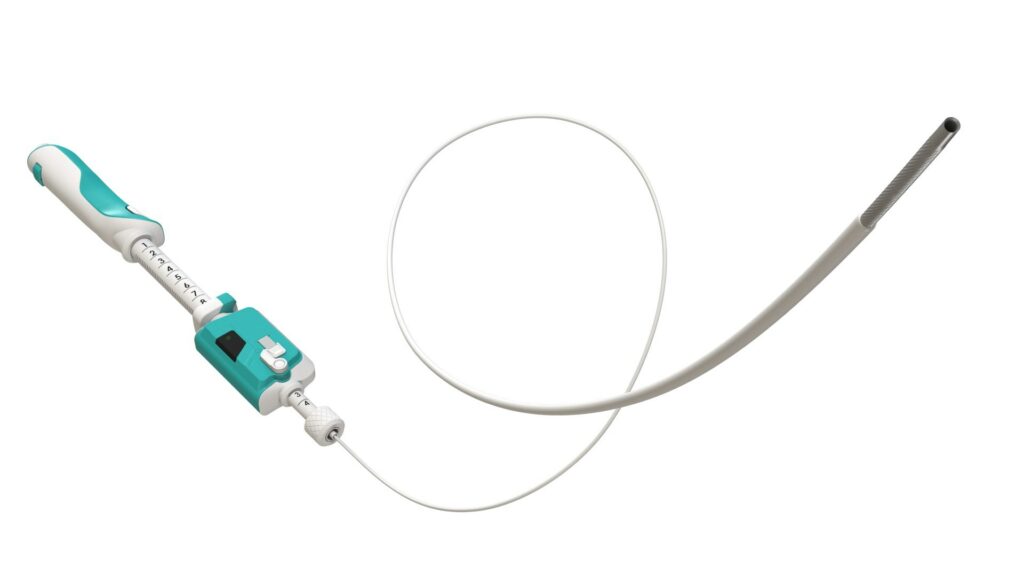
Limaca Medical (“Limaca”) announced that its Precision-GI™ Endoscopic Ultrasound Biopsy Product has received a Breakthrough Device Designation from the U.S. Food and Drug Administration (“FDA”).
The Precision-GI™ device is designed to obtain tumor tissue within or adjacent to the gastrointestinal tract. Endoscopic biopsy is performed by a gastroenterologist who accesses the targeted GI tumor utilizing an ultrasound visualization endoscope. Precision-GI™ is deployed and operated through an instrument channel in the endoscope to biopsy the tumor. Suspect GI tumor locations include submucosal lesions, mediastinal masses, lymph nodes, intraperitoneal masses, and within GI related organs such as the pancreas and liver.
While all existing endoscopic ultrasound fine needle biopsy (EUS-FNB) devices require manual hand operation, Precision GI™ features a unique motorized, automated rotational cutting needle for successful tissue acquisition. Today’s endoscopic biopsy devices have limitations in consistently obtaining quality tissue with sufficient quantity, which can result in sample tissue fragmentation, inadequate tissue amount, and blood contamination.
Precision GI™ is designed to obtain biopsies for definitive diagnosis of pancreatic cancer and other life-threatening GI cancers more quickly and less traumatically than current products. The automated design will provide for more efficient and effective diagnosis of GI cancers since it is designed to yield significantly superior quality and quantity of diagnostically relevant biopsy tissue.
“Precision-GI™ is an automated, motorized endoscopic biopsy product that has the potential to improve our biopsy results for the evaluation of gastrointestinal malignancies. Endoscopic biopsy is a highly specialized, high skill procedure. We welcome the innovation of Precision-GI™ which can provide automation and standardization of outcomes with less variation from operator to operator,” stated Seth A. Gross, MD, Clinical Chief, Division of Gastroenterology and Hepatology, NYU Langone Health. “Our field is driving toward patient centric individualized cancer therapy, known as Precision Medicine, which requires consistently high quality and quantity of endoscopic biopsy tissue, enabling optimal matching of the tumor’s genetic profile to personalize a patient’s treatment plan.”
“We are pleased with the FDA’s decision to grant the Breakthrough Device Designation to Precision-GI™,” stated Carl Rickenbaugh, Limaca’s CEO. “At Limaca, our vision is to ensure that endoscopic biopsies always achieve a definitive diagnosis to enable optimal and timely GI-cancer treatment. We are dedicated to the mission to provide a far better endoscopic biopsy experience for the endoscopist and patient, with the goal to achieve a faster, more efficient biopsy yield with highly consistent results. With the Breakthrough Device Designation, we look forward to accelerating our progress toward our goal of obtaining the FDA’s 510(k) clearance to bring Precision-GI™ to patients in the US in the near future.”
The FDA Breakthrough Device Designation expedites the development and evaluation of novel devices that offer the potential to enhance patient outcomes through more effective treatment or improved diagnosis of life-threatening conditions. Under the program, the FDA will provide Limaca with the opportunity to provide its feedback during the pre-market phase and prioritized review of the device submission.
About Limaca Medical
Limaca Medical, Ltd, is a privately held company based in Israel that is dedicated to improving endoscopic biopsy results for patients facing potentially life threatening gastroenterology cancers. Limaca has an experienced team of engineers, clinicians, and business professionals with successful track records of innovating, developing, and commercializing specialized medical devices. Limaca Medical is primarily funded by the Israeli Innovation Authority, Agriline (a trust of which Vincent Tchenguiz is a discretionary beneficiary), and The Trendlines Group, Ltd (SGX:42T;OTCQX:TRNLY), an innovation commercialization company that invents, discovers, invests in, and incubates innovation-based medical and agricultural technologies to fulfil its mission to improve the human condition.
See Full Press Release at the Source: Limaca Medical Receives FDA Breakthrough Device Designation
Press Release by: Limaca Medical
Legacy MedSearch has more than 35 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 17 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.