Medical device and MedTech insights, news, tips and more
Masimo Announces FDA Clearance for Neonatal RD SET Pulse Oximetry Sensors with Improved Accuracy Specifications
December 30, 2019

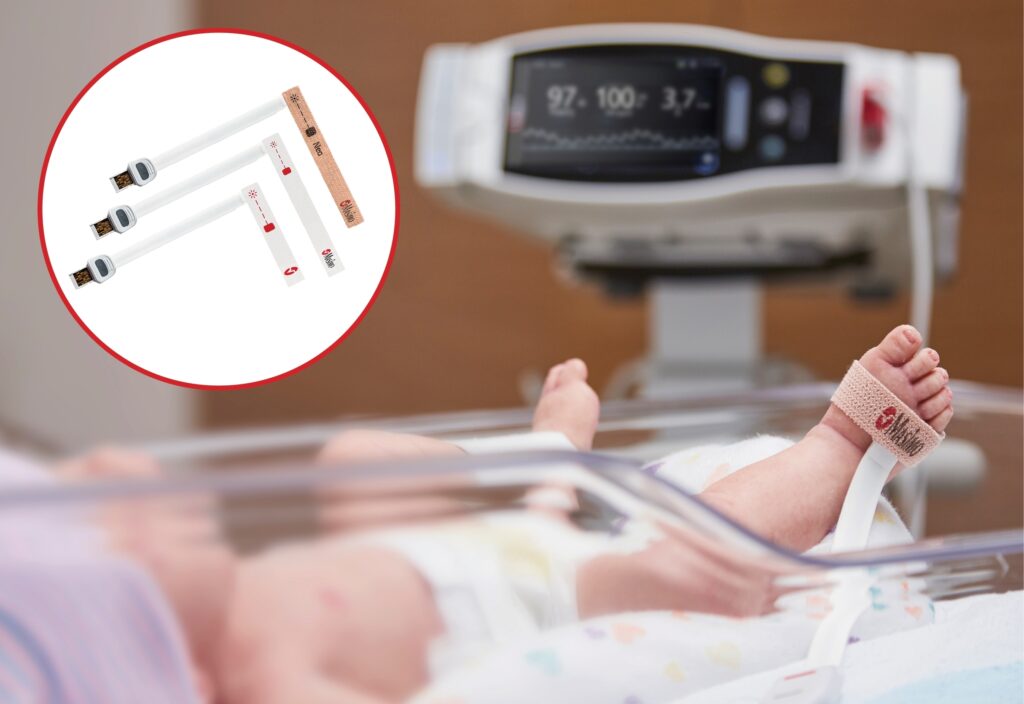
Masimo announced today that RD SET® sensors with Masimo Measure-through Motion and Low Perfusion™ SET® pulse oximetry have received FDA clearance for improved oxygen saturation (SpO2) accuracy specifications for neonatal patients (< 3 kg). The updated RD SET® sensors’ SpO2 accuracy specifications have improved significantly, from 3% to 1.5% ARMS1 (at 1 standard deviation), in conditions of motion and no motion, providing clinicians with even greater confidence when monitoring the oxygenation status of neonates. With this clearance, the improved performance specifications, which were incorporated into RD SET® sensors for patients > 3 kg in 2018, are now available to all patient populations in the United States.
Masimo’s revolutionary SET® pulse oximetry has been shown in more than 100 independent and objective studies to outperform other pulse oximetry technologies – even before the revisions that achieved the improved accuracy specifications – providing clinicians with increased sensitivity and specificity to help them make critical patient care decisions.2 Crucially for newborn health, SET® has been shown to help clinicians reduce severe retinopathy of prematurity in neonates3 and in multiple studies, including the largest critical congenital heart disease (CCHD) study to date, to improve CCHD screening in newborns.4-5
In addition to offering improved accuracy, RD SET® sensors are designed to enhance patient comfort, optimize clinician workflows, and help hospitals meet green initiatives. The sensors are lightweight and have a flat, soft cable with smooth edges, so that they lie comfortably on a patient’s hand or foot. In particular, RD SET® NeoPt sensors with Velaid SofTouch™ use little to no adhesive, facilitating quick but gentle application and repositioning on the fragile skin of newborns and pre-term babies. RD SET® sensors also feature an intuitive sensor-to-cable connection, while their lightweight design results in up to 84% less waste and their sleek, recyclable packaging reduces storage and shipping space.
Joe Kiani, Founder and CEO of Masimo, said, “We’re delighted to announce the latest result of our continued innovation in our foundational SET® pulse oximetry. We have long been dedicated to helping improve the lives of neonatal, infant, and pediatric patients, and this clearance significantly furthers that mission. Thanks to the brilliance and dedication of our engineers and the continuing support of our customers, we’ve been able to once again raise the standard for pulse oximetry performance. Even though no one has been able to create pulse oximetry that outperforms SET®, we have not allowed that to stop us from continuing our pursuit of perfecting pulse oximetry.”
See Full Press Release: Masimo Announces FDA Clearance for Neonatal RD SET® Pulse Oximetry Sensors with Improved Accuracy Specifications | Business Wire
Written by: Masimo

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.