Medical device and MedTech insights, news, tips and more
Medtronic Announces First U.S. Spine Patients Treated with Mazor X Stealth Robotic Surgical System
January 28, 2019
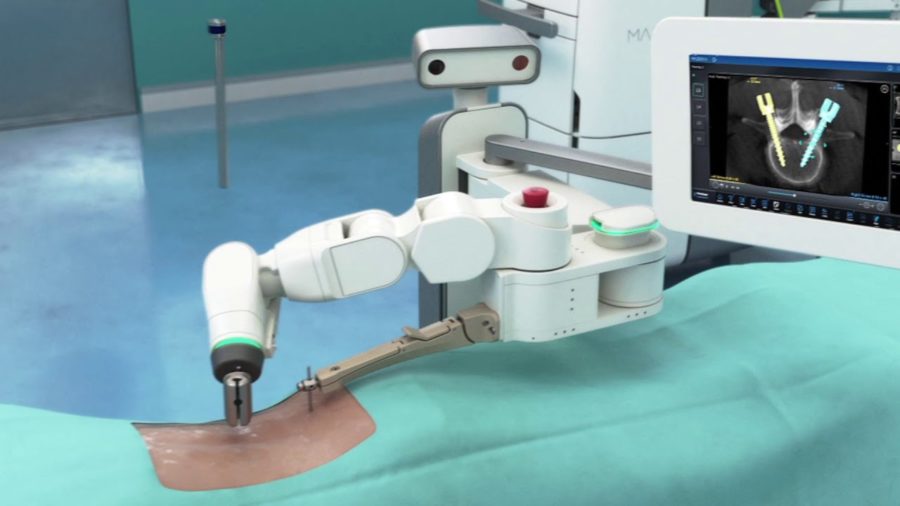
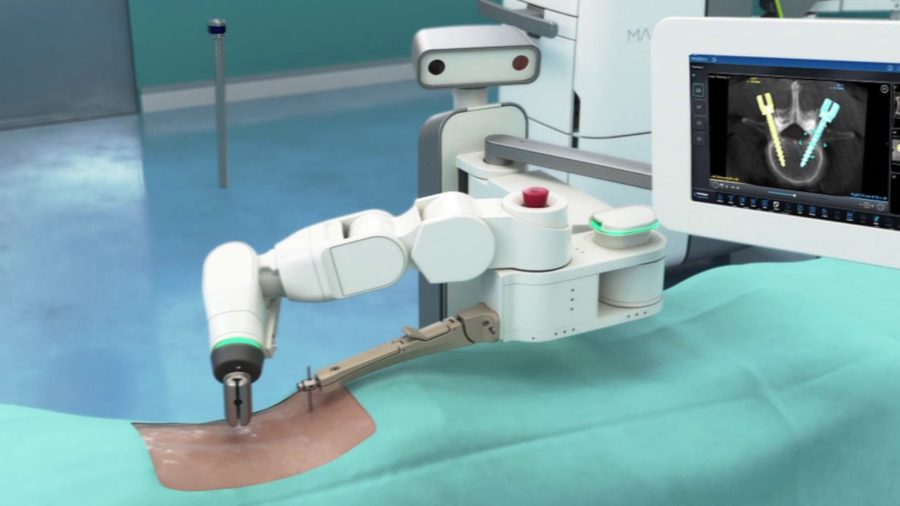
Medtronic today announced the first U.S. patients treated with the Mazor X Stealth Edition for spine surgery following its recent commercial launch. The Mazor X Stealth Edition offers a fully-integrated procedural solution for surgical planning, workflow, execution and confirmation. The system was first used at Norton Healthcare in Louisville, Ky., and Reston Hospital Center in Reston, Va.
“It is rare that huge sectors of technology such as robotics and navigation merge into a sole platform as found in the Mazor X Stealth Edition. Combined, these may provide more predictable and reliable execution of our procedure workflows,” remarked Jeffrey Gum, M.D., orthopedic spine surgeon at Norton Leatherman Spine. “Computerized surgical planning, 3-D assessment of spine anatomy, robotic guidance and live navigation feedback are designed to provide a higher degree of accuracy throughout the surgical procedure.”
Co-developed between Medtronic and the recently acquired Mazor Robotics, the Mazor X Stealth Edition seamlessly incorporates Stealth(TM) software technology into the Mazor X(TM) robotic-assisted surgery platform to deliver workflow predictability and flexibility through real-time image guidance, visualization and navigation informed by interactive 3-D planning and information systems.
“The marriage of robotics and navigation represents the future of computerized planning and execution in spine surgery. Robotics and navigation have both been shown to improve accuracy and precision in spine surgery,” commented Christopher R. Good, M.D. F.A.C.S., spine surgeon at Reston Hospital Center, director of Scoliosis & Spinal Deformity and president of The Virginia Spine Institute. “The Mazor X Stealth Edition is a revolutionary new technology that uses cutting-edge software to plan the surgical procedure, then uses a robotic arm to guide implants and instruments through the steps of the surgical procedure with precision, while simultaneously using real-time imaging feedback to ensure the plan is being carried out as desired.”
The Mazor X Stealth Edition was cleared by the U.S. Food and Drug Administration (FDA) in November 2018. The system is now available in the U.S. and is expected to launch in key regions throughout the year.
“As part of our Surgical Synergy strategy, we believe Mazor X Stealth Edition will accelerate the advancement and adoption of robotic-assisted and navigated surgical technologies in spine,” said Geoff Martha, executive vice president and president of the Restorative Therapies Group at Medtronic. “Medtronic is committed to transforming the future of spine care by offering procedural solutions that integrate implants, biologics and enabling technologies like navigation, 3-D imaging, robotics and powered surgical tools.”
Press Release by Medtronic
 A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.