Medical device and MedTech insights, news, tips and more
Medtronic Receives FDA Approval for CRT Defibrillator That Improves Pacing
November 28, 2016
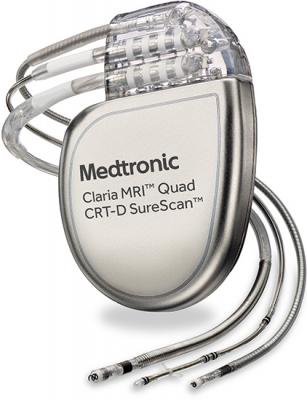
November 15, 2016 — Medtronic plc has received U.S. Food and Drug Administration (FDA) approval for the Claria MRI Quad Cardiac Resynchronization Therapy Defibrillator (CRT-D) SureScan device for patients with heart failure. The Claria MRI CRT-D is approved for scanning in both 1.5 and 3 Tesla (T) magnetic resonance imaging (MRI) machines, and features EffectivCRT, a new algorithm that automatically tailors the therapy to individual patients by adjusting pacing rates.
“Until now, CRT devices have shown only whether a pacing pulse was sent, but we haven’t been able to determine if that stimulation actually improves the heart’s pumping ability,” said Suneet Mittal, M.D., director, electrophysiology laboratory, Arrhythmia Institute of the Valley Health System, Ridgewood, N.J. “With the Claria device, physicians are now able to verify the effectiveness of left ventricular pacing, which is especially beneficial for improving outcomes in patients with atrial fibrillation, who have been difficult to treat because this irregular and rapid heartbeat often interferes with the delivery of effective CRT.”
A large percentage of heart failure patients receiving cardiac resynchronization therapy have atrial fibrillation (AF), which can significantly reduce patient response to CRT. The Claria device includes the Medtronic-exclusive EffectivCRT Diagnostic, which automatically determines the effectiveness of each left ventricular pace, and the EffectivCRT during AF algorithm, which automatically adjusts pacing rates during AF, without adversely affecting the average heart rate.
Additional features on the Claria device include:
- The AdaptivCRT algorithm, which reduces a patient’s odds of a 30-day heart failure readmission by 59 percent, and has demonstrated a 46 percent reduction in AF risk compared to echo-optimized biventricular pacing
- VectorExpress 2.0, an automated in-office test that reduces lead programing to two minutes, and reveals clinically actionable information to help physicians select optimal pacing configurations for each patient;
- Attain Perfoma MRI SureScan Quadripolar Leads, which include short bipolar spacing to reduce phrenic nerve stimulation occurrence, steroid on all electrodes, and three shapes for varying patient anatomies; and
- SureScan MR-conditional labeling for full-body scans without positioning restrictions. Medtronic now offers MR-conditional pacemakers, implantable cardioverter defibrillators (ICDs), insertable cardiac monitors (ICMs) and CRT-Ds. Additionally, patients with certain existing defibrillation leads will be eligible for an MR-conditional ICD or CRT-D, and thus able to access this important imaging technology.
Medtronic also has submitted a Pre-Market Application (PMA) to the FDA for Multiple Point Pacing, which, if approved, would be available with the Claria MRI and Amplia MRI CRT-Ds.
For more information: www.medtronic.com
Read Full Article – Source: Medtronic Receives FDA Approval for Claria MRI Quad Cardiac Resynchronization Therapy Defibrillator | Diagnostic and Interventional Cardiology
