Medical device and MedTech insights, news, tips and more
Minnetronix Medical Wins FDA Clearance for Innovative Neurosurgical Access Platform
September 10, 2020
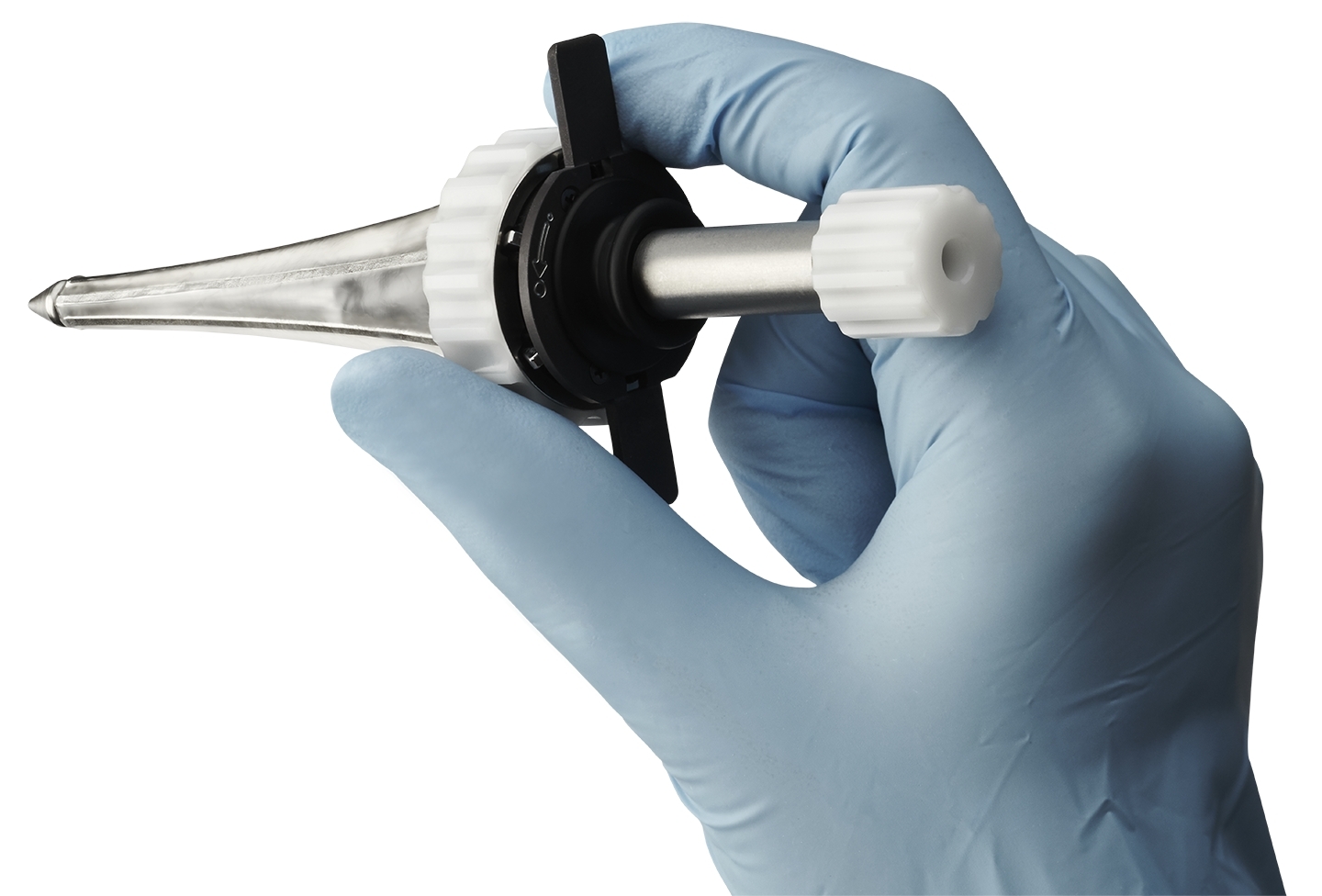
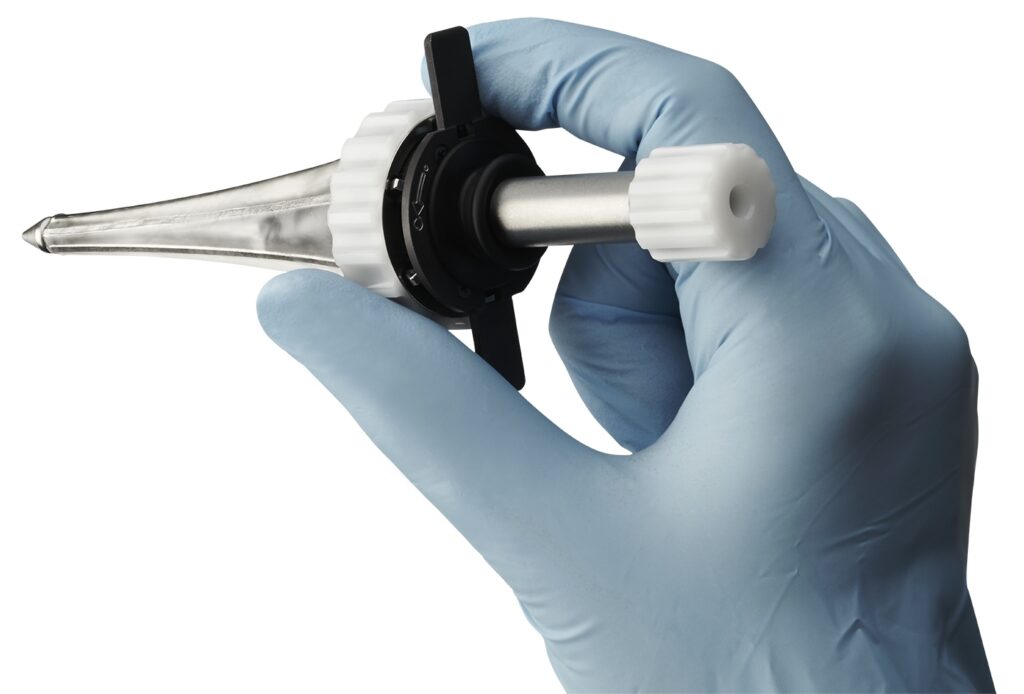
Minnetronix Medical, the company known for 25 years of developing and manufacturing products for medical device companies throughout the world, today announced that it has received FDA clearance for its first platform product: the MindsEye™ Expandable Port for neurosurgical procedures. This clearance represents an expansion of the company’s traditional offerings to include market-ready platforms.
The MindsEye Port is the first minimally invasive, expandable port designed for use in the treatment of stroke, cancer, and other conditions. The device’s dynamic retraction creates a custom-sized channel allowing surgeons to reach target areas deep within the brain. “This is next-generation deep brain access technology,” said Dr. Mario Zuccarello, Professor of Neurosurgery, University of Cincinnati Medical Center. “Minimizing invasiveness as much as possible is important to respect the eloquence of brain tissue. The MindsEye Expandable Port allows surgeons to work without unnecessary distractions, which ultimately improves quality of life for the patient.”
“Given its unique features, the port has already attracted interest among neurosurgeons and potential distribution partners. They’re excited to get their hands on it,” says Matt Adams, vice president and general manager at Minnetronix Medical. “The MindsEye Port is an exciting example of how Minnetronix Medical is expanding its offerings by developing market-ready products.” He adds that the port represents an entire platform for the company, as it has additional applications and complimentary product opportunities in the neuro market and beyond.
About Minnetronix Medical
Since 1996, Minnetronix Medical has accelerated medical device breakthroughs via design, development and manufacturing services for companies around the world. The experienced Minnetronix team works side-by-side with customers to successfully navigate increasingly complex medical device development and commercialization in the fluid and gas management, optical systems, RF/EM energy, and stimulation and active wearables markets. The company is currently developing a portfolio of solutions that advance treatment options, prevent secondary injury and enhance healing for patients in the Neuro ICU. Minnetronix Medical is based in St. Paul, Minn.
See Full Press Release at the Source: Minnetronix – Minnetronix Medical Gets FDA Clearance on Innovative Neurosurgical Access Platform
Press Release by: Minnetronix Medical
 Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.