Medical device and MedTech insights, news, tips and more
Miracor’s Heart Attack Device Secures FDA Breakthrough Status
August 23, 2019
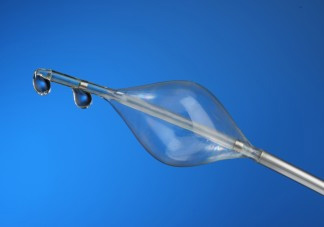
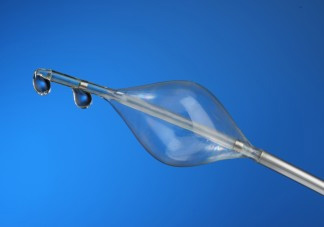
The concept of pressure-controlled intermittent coronary sinus occlusion, abbreviated to PICSO, has been around since the 1980s. PICSO entails placing a device in the coronary sinus to intermittently obstruct blood flow. Through the interference, PICSO may increase blood flow to parts of the heart affected by the myocardial infarction.
Miracor, which received a CE mark for its PiCSO system in 2010, built on the idea by developing an algorithm to calculate how long to block venous drainage via the coronary sinus.
The breakthrough device designation marks a step forward in Miracor’s efforts to bring the product to the U.S. market. Under the breakthrough program, Miracor stands to benefit from more frequent interactions with FDA and, if it makes it to the filing stage, an expedited regulatory review.
Miracor is still building out the evidence in support of its device. A first-in-human trial published in 2012 showed problems, mainly initial calibration difficulties, prevented the use of its system in one-third of the enrolled patients.
A subsequent study was stopped “due to slow enrollment and a relatively high rate of unsuccessful PiCSO procedures.” Again, around one-third of patients were unable to undergo the procedure for a variety of reasons. Among the 19 patients who did receive the treatment, infarct size and myocardial recovery were not significantly better than among a historical control group.
Miracor recently started a larger clinical trial that is randomizing patients to either receive its system or standard percutaneous coronary intervention. The trial is designed to show whether using the device in addition to PCI reduces infarct size at five days compared to PCI alone.
See Full Article at the Source: Heart attack device earns FDA’s breakthrough nod | MedTech Dive
Article Written by: Nick Paul Taylor
 A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
A Speciality Recruiting Firm Exclusively Servicing The Medical Device IndustryLegacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.