Medical device and MedTech insights, news, tips and more
Neocis Announces FDA 510(k) Clearance of Additional Drill for Use with Yomi®
October 1, 2019
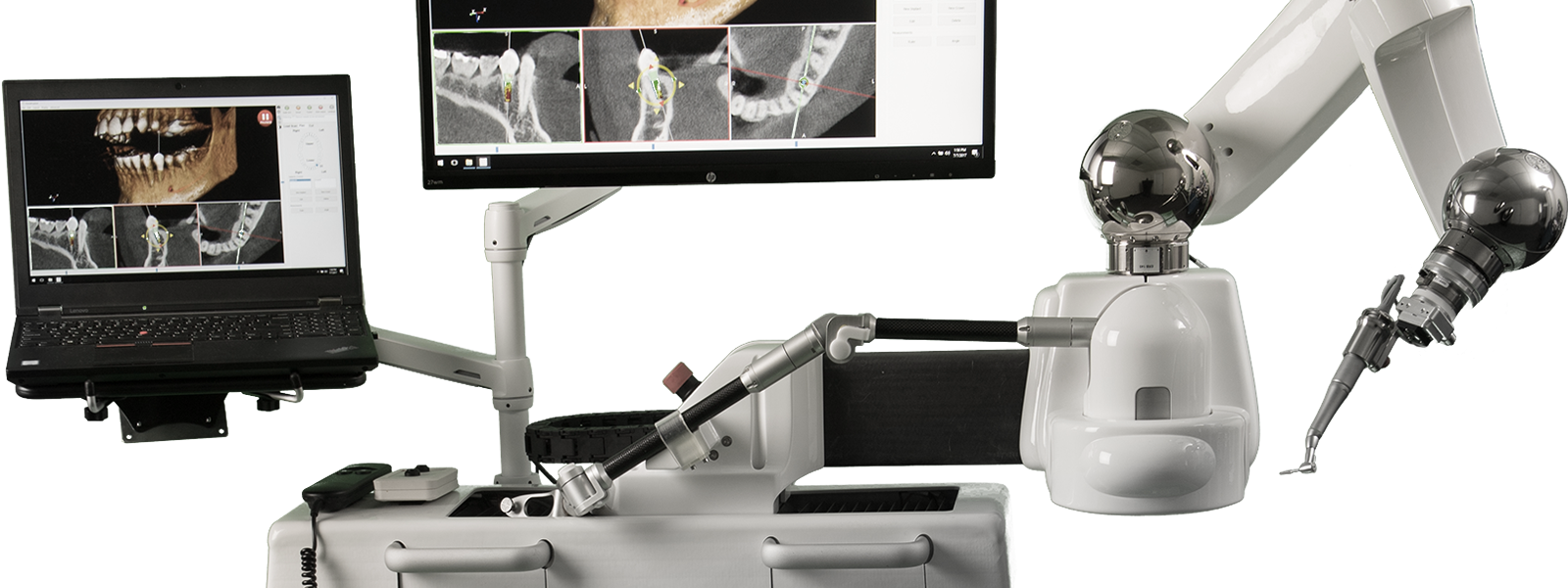
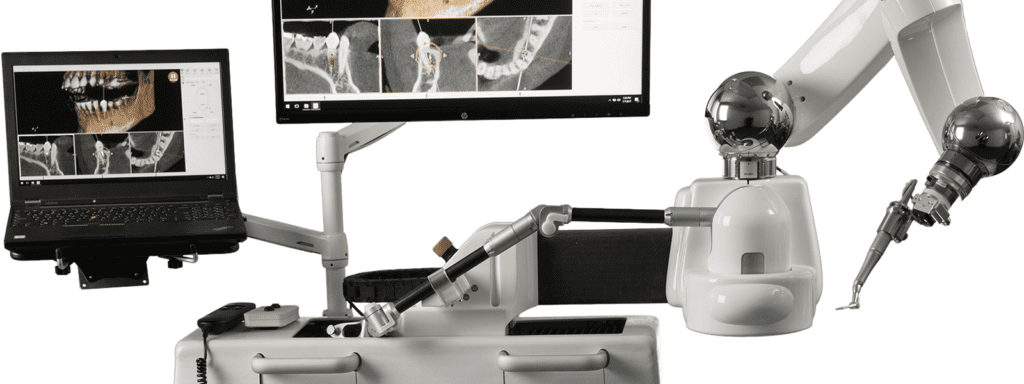
Neocis, Inc., the leader and innovator in dental implant surgery using advanced robotics, today announced a 510(k) clearance from the U.S. Food and Drug Administration (FDA) for a new dental drill for use with Yomi. This new clearance leads to an easier workflow and provides enhancements to the surgeons’ drilling experience.
Yomi, the first and only FDA cleared robot-assisted dental surgery system, provides computerized navigation to assist in both the planning (pre-operative) and the surgical (intra-operative) phases of dental implantation surgery. The system offers physical guidance through haptic robotic technology, which constrains the drill in position, orientation, and depth. The assistive technology provides the surgeon with complete control and, unlike plastic surgical guides, allows for clear visualization of the surgical site.
“Streamlining the workflow for our robot-assisted surgery system is the most critical part of enabling this technology to be broadly adopted,” said Alon Mozes, PhD, Chief Executive Officer of Neocis. “We are laser-focused on creating a great experience for the surgeon and the patient.”
Dr. Scotty Bolding, a nationally recognized leader in Oral and Maxillofacial Surgery, says, “I am tremendously impressed with the team at Neocis. They are truly listening to the professionals and continue to advance the robotic technology to make the system more accurate and user-friendly. This latest FDA clearance is just another example of how they are making the system easier to use and making YOMI not only routine in our practice, but establishing the standard of care for our patients.”
About Neocis, Inc.
Neocis® is a private company located in Miami, Florida that is transforming dental implant surgery with advanced robotics, with a vision of advancing healthcare through the latest technology. The company’s FDA cleared Neocis Guidance System, or Yomi®, is indicated for use to provide assistance in both the planning (pre-operative) and the surgical (intra-operative) phases of dental implantation surgery. The system provides software to preoperatively plan dental implantation procedures and provides navigational guidance of the surgical instruments. Yomi® provides surgical guidance through the use of haptic robotic technology, software, and multisensory feedback to help achieve the right position, angulation, and depth to place the implant exactly according to plan. Yomi® enables a minimally invasive flapless approach, which has been proven to lead to faster surgery, faster recovery, and less pain for the patient.
See Full Press Release: Neocis Announces FDA 510(k) Clearance of Additional Drill for Use with Yomi® | Neocis
Written by: Neocis

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.