Medical device and MedTech insights, news, tips and more
NeuroOne® Receives FDA 510(k) Clearance to Market its Evo® sEEG System for Less than 30 Day Use
October 25, 2022
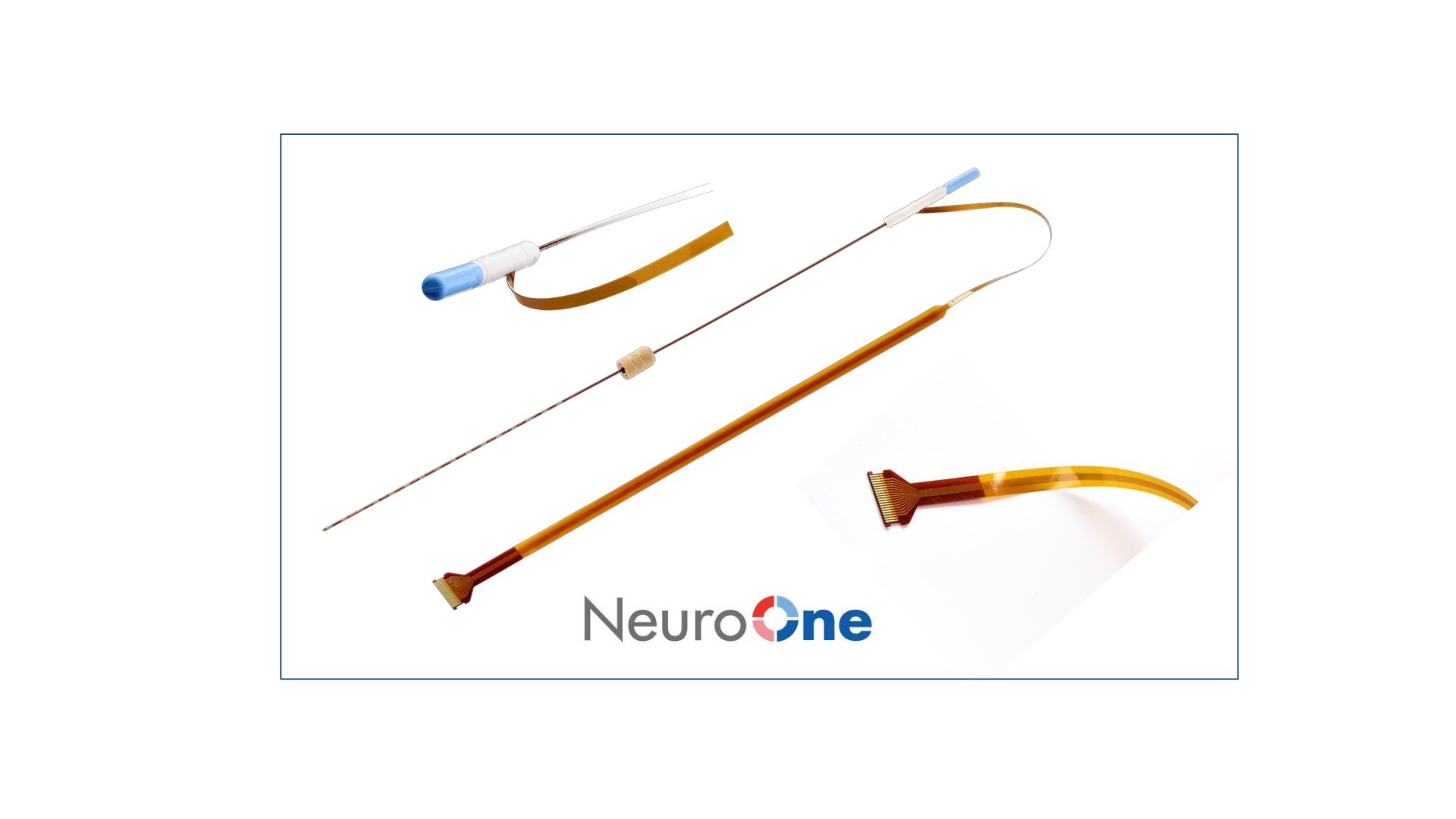
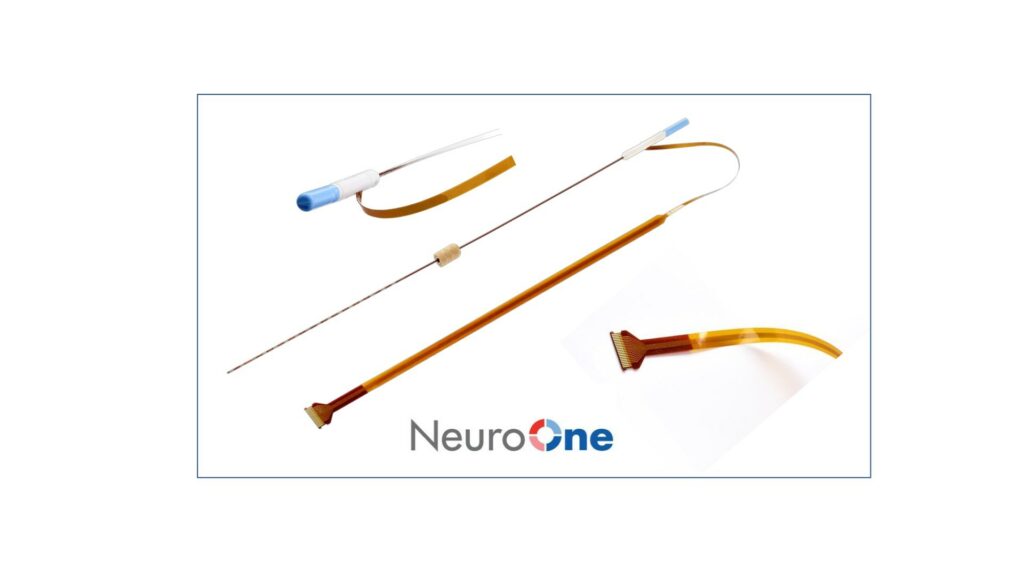
NeuroOne Medical Technologies Corporation, a medical technology company focused on improving surgical care options and outcomes for patients suffering from neurological disorders, announced that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance to market its Evo sEEG Electrode technology for temporary (less than 30 days) use with recording, monitoring, and stimulation equipment for the recording, monitoring, and stimulation of electrical signals at the subsurface level of the brain.
On August 9, 2022, the Company announced it had resubmitted its 510(k) application to the FDA for less than 30 day use, which included additional biocompatibility testing as requested by the FDA. On October 20, 2022, the Company received an FDA clearance letter stating that the Evo sEEG System is substantially equivalent to the predicate device and may be marketed in the United States.
“I am extremely proud of the entire NeuroOne team and their relentless pursuit of this clearance. Despite the challenges we faced, our team remained focused and persistent in driving this successful conclusion. This is clearly our most exciting and important accomplishment to date. We are now able to advance our commercialization efforts in partnership with Zimmer Biomet, our distribution and development partner. We look forward to continuing to execute our strategic plan, which next up includes our RF ablation system, the Company’s first therapeutic electrode technology,” said Dave Rosa, CEO of NeuroOne.
The Evo sEEG System represents the Company’s second FDA 510(k) cleared product. NeuroOne now provides a full line of electrode technology to address an estimated worldwide market of $100 million for patients requiring diagnostic brain mapping procedures. As opposed to cortical electrodes, sEEG electrodes provide a similar function at the subsurface level of the brain by using a much less invasive process that does not require removal of the top portion of the patient’s skull. sEEG electrodes are the predominant technology used in these procedures due to their less invasive placement and subsurface location.
The Company’s Evo Cortical and sEEG Electrodes are a portfolio of hi-definition thin film electrodes. Potential advantages include increased signal clarity and reduced noise; better tactile feedback during insertion into brain tissue; and faster order fulfillment due to an automated manufacturing process.
As previously reported, NeuroOne is also advancing a pipeline of therapeutic electrode technologies for brain tissue ablation and chronic stimulation use for DBS (deep brain stimulation) and spinal cord stimulation for chronic back pain. These therapeutic electrode technologies represent addressable markets valued between $500 million and $6 billion.
About NeuroOne
NeuroOne Medical Technologies Corporation is a developmental stage company committed to providing minimally invasive and hi-definition solutions for EEG recording, brain stimulation and ablation solutions for patients suffering from epilepsy, Parkinson’s disease, dystonia, essential tremors, chronic pain due to failed back surgeries and other related neurological disorders that may improve patient outcomes and reduce procedural costs. For more information, visit https://www.n1mtc.com.
See Full Press Release at the Source: NeuroOne® Receives FDA 510(k) Clearance to Market its Evo® sEEG System for Less than 30 Day Use
Press Release by: NeuroOne Medical Technologies Corporation
Legacy MedSearch has more than 35 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 17 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.