Medical device and MedTech insights, news, tips and more
New Tools for Preventing Hospital-Acquired Infections
October 24, 2016
As the focus on hospital-acquired infections intensifies, some medical device manufacturers are considering deep UV light emitting diodes for smaller, portable disinfection devices. Here’s how the technology can be used in medical device design.
According to the Centers for Disease Control and Prevention (CDC) one in every 25 hospital patients in the United States will contract a hospital-acquired infection (HAI) during their visit. Often expensive and sometimes fatal, HAIs are adding billions to the country’s already skyrocketing healthcare costs.
For decades, hospitals and other healthcare facilities have relied on ultraviolet germicidal irradiation (more recently referred to as germicidal UV, or GUV) systems equipped with low pressure mercury vapor lamps to disinfect everything from operating rooms to the air circulating in the building’s ductwork.
As an alternative, medical device manufacturers are turning to deep UV (UVC) light emitting diodes (LEDs), which offer a much smaller footprint as well as UVC light in the optimal 250-280 nm wavelength range. While the small footprint provides designers with unparalleled flexibility, they can also adjust the LED’s input drive current to optimize the light intensity and create customized disinfection tools that directly support and improve a healthcare facility’s HAI prevention protocols. For the most part, these systems provide an increased level of disinfection. However, low-pressure mercury lamps emit light at a spectral peak wavelength of 253.7 nm. Though effective, the wavelength is not optimal from a germicidal efficiency standpoint. More importantly, due to their size and fragility, the lamps cannot address the healthcare industry’s growing need for smaller, portable UV disinfection devices.
Developing New, Portable Solutions
Consistent with the growing shift from managed care to a more decentralized model, leading medical device manufacturers are developing innovative new solutions to safeguard both patients and clinicians from harmful drug-resistant pathogens and life threatening infections. At the end of 2015, the newly signed federal budget temporarily suspended the 2.3% excise tax on medical devices for the next 24 months, in part allowing device manufacturers, both public and private, to resume R&D programs.
Ultraviolet (UV) disinfection is a reliable and viable alternative to chemical disinfection methods because it disrupts the DNA of harmful microorganisms and destroys their ability to reproduce—thereby eliminating the spread of MRSA, C. difficile (C. diff), staph, and many other pathogens. The C. diff spore, which can remain active on surfaces for up to three months and is the most difficult to kill, is generally regarded as the benchmark for confirming new disinfection process efficacy.
From a system or portable instrument perspective, UVC LEDs are a major upgrade. They can deliver UVC light in optimal wavelengths for targeted disinfection and they have a much smaller footprint, consuming far less energy due to their instant on/off operation. As a result, designers are able to develop new and increasingly portable GUV disinfection instruments that can do a better job preventing the spread of infection at the hospital, community care facilities, or in the home.
Variation in Required UV Dosage by Microbe
UV disinfection relies on radiation emitted in the wavelength range of 250 nm to 280 nm to inactivate pathogens by breaking their DNA strand. Following a number of important advancements in semiconductor-based UVC LED technology, LEDs are now viewed as a viable, cost-effective, and environmentally friendly alternative to mercury-based lamp technologies.
As interest in UVC LEDs continues to grow, medical device/system designers looking to develop new products must first focus on the log reduction target to optimize the UV dosage for their application. Log reduction is a mathematical term used to show the relative number of live microbes that are eliminated from a surface by disinfecting or cleaning.
For example, a “4-log reduction” lowers the number of microorganisms by 10,000-fold—if a surface contains 10,000 pathogenic microbes, a 4-log reduction would reduce the number of microorganisms to one.
By establishing the log reduction target for an application first, designers can then determine the distance from the light source and time of exposure that’s needed to achieve it. Or, to put it another way, the distance from the UVC energy, combined with the amount of time the surface is exposed to the UVC light, will directly determine the level or degree of disinfection.
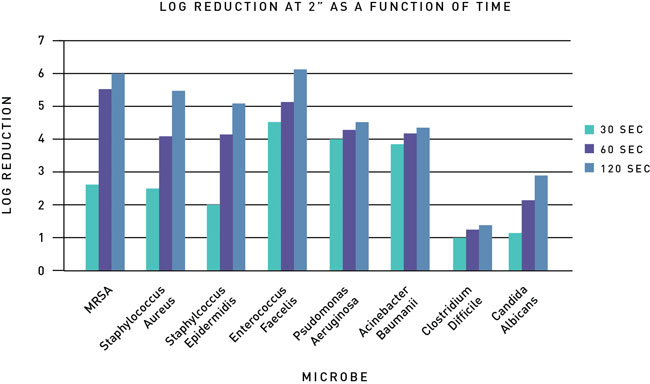
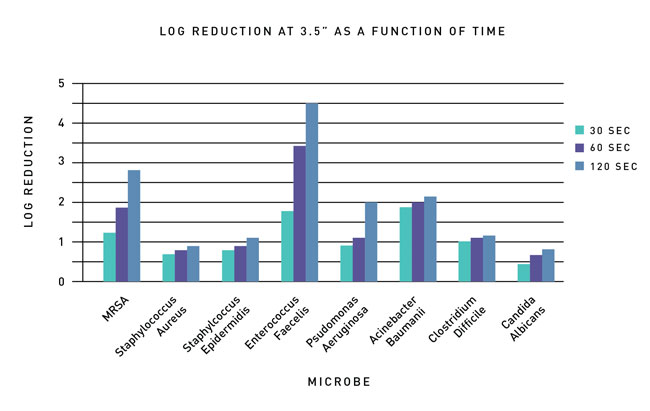
Experiments recently conducted at EMSL, a third-party microbiological testing facility in New York, targeted common microbes for UVC LED disinfection applications (Table 1). The goal was to measure the level of disinfection achieved on different microbes across a range in gram positive, gram negative, and fungi groups as a function of time and distance.
| Microbe | Type |
| MRSA | Gram + Bacteria |
| Staphylococcus aureus | Gram + Bacteria |
| Staphylococcus epidermidis | Gram + Bacteria |
| Enterococcus faecelis | Gram + Bacteria |
| Pseudomonas aeruginosa | Gram – Bacteria |
| Acinebacter baumanii | Gram – Bacteria |
| Clostridium difficile | Gram + Bacteria (spore forming) |
| Candida albicans | Fungi |
The log reduction for each microbe was measured after being exposed to UV at intervals of 30, 60, and 120 seconds. The exposure data can be seen in Figures 1 and 2 at distances from the light source of 2 inches and 3.5 inches respectively. After 120 seconds of exposure at a distance of 2 inches, there was slightly more than a 5.3 log reduction on all the gram positive bacteria with a 6 log reduction for MRSA. Bacteria of gram negative types experience a log reduction value between 4 and 5; fungi had a log reduction value of approximately 3 and gram positive (spore forming) experienced just over a 1 log reduction.
Read Full Article – Source: New Tools for Preventing Hospital-Acquired Infections | MDDI Medical Device and Diagnostic Industry News Products and Suppliers
Author – Mark Pizzuto