Medical device and MedTech insights, news, tips and more
Nipro introduces SURDIAL™ DX Hemodialysis System to U.S. Market
March 29, 2022
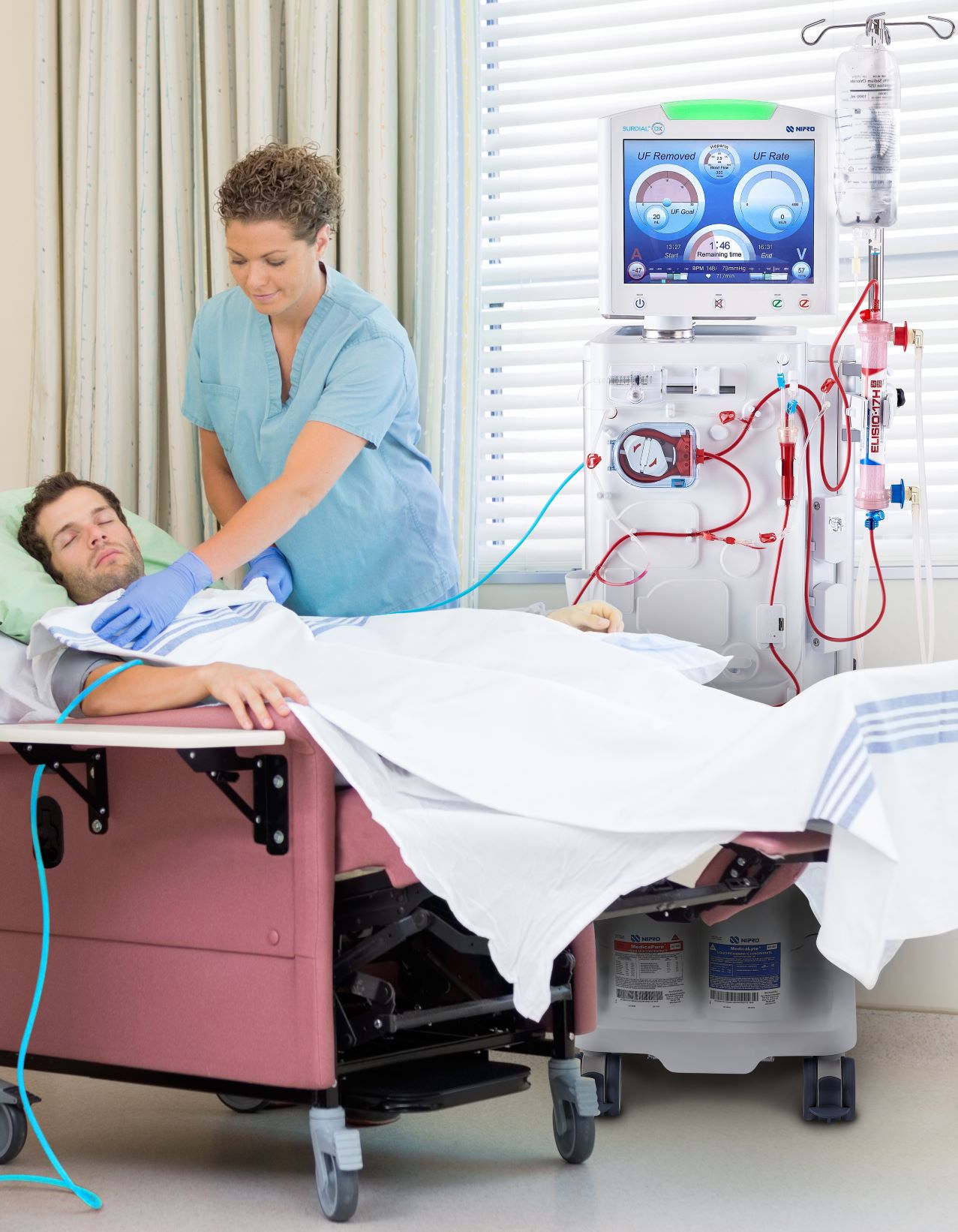
Nipro Medical Corporation (Nipro), a leading manufacturer and supplier of renal, vascular, and medical-surgical products, announced the commercial launch of SURDIAL™ DX Hemodialysis System to the U.S.
SURDIAL™ DX is a state-of-the-art hemodialysis system designed to create an optimal dialysis treatment experience for patients and clinicians. Manufactured in Japan, it draws from over 35 years of expertise in renal device innovation at Nipro’s parent company, Nipro Corporation.
SURDIAL™ DX was developed with key input from clinicians to offer patient-focused features for optimal performance, efficiency, and ergonomics. With its soft launch in late 2021, the U.S. team proudly rolls out the full-scale commercial release of SURDIAL™ DX to dialysis clinics nationwide.
Foundational to SURDIAL™ DX:
- patient safety and comfort
- ease of use and step-by-step guidance
- streamlined automated processes
- ergonomic design
- supported by continuous clinical education
In addition to the machine’s performance and features, Nipro offers comprehensive services and an extensive training certification curriculum to support customers and to differentiate themselves among current dialysis systems in the U.S. Hence, Nipro’s invitation to “experience the difference” with SURDIAL™ DX for healthcare professionals and patients alike, and by making it “truly a ‘white glove’ experience,” says Renata Zugaj, Director of the Medical Instrument Center in North America who led the project.
“SURDIAL™ DX is in full alignment with Nipro’s long-term strategic initiative: to be America’s comprehensive solution provider for hemodialysis,” commented Joe Dawson, Executive VP of Nipro Medical Corporation. With local manufacturing in Pennsylvania and supported by a global network of 60+ production sites, Nipro is indeed well-positioned to supply a full-scale renal portfolio to Americans – coupling SURDIAL™ DX together with their line of dialyzers, blood tubing sets, AVF needles, concentrates, solutions, vascular access management, and other renal accessories.
About Nipro Medical Corporation:
Nipro Medical Corporation – with offices in Bridgewater, NJ and Miami, FL – is a wholly-owned subsidiary of Nipro Corporation Japan, headquartered in Osaka. Established 1954, Nipro is a world-leader in healthcare with over 33,000 employees, 60 production sites, and 240 sales offices spanning 6 continents.
Nipro’s North America division is responsible for managing the sales, marketing, and business operations of the United States and Canada – continuously committed to improving patient care and quality of life by developing safe, high-quality, easy to use, and effective solutions that meet daily clinical objectives and reimbursement needs.
See Full Press Release at the Source: Nipro introduces SURDIAL™ DX Hemodialysis System to U.S. market, inviting dialysis patients and clinicians to “experience the difference.”
Press Release by: Nipro
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.


