Medical device and MedTech insights, news, tips and more
Omega Medical Imaging First in the World to Receive FDA Clearance on Artificial Intelligence Imaging System that Reduces Radiation Exposure
November 7, 2019
Omega Medical Imaging, manufactures of Artificial Intelligence Fluoroscopy/Cine (AIF/C) Imaging systems, just announced the Food and Drug Administration 510 (k) clearance of FluoroShield™ with their 2020 Cardiac Flat Panel Detector.
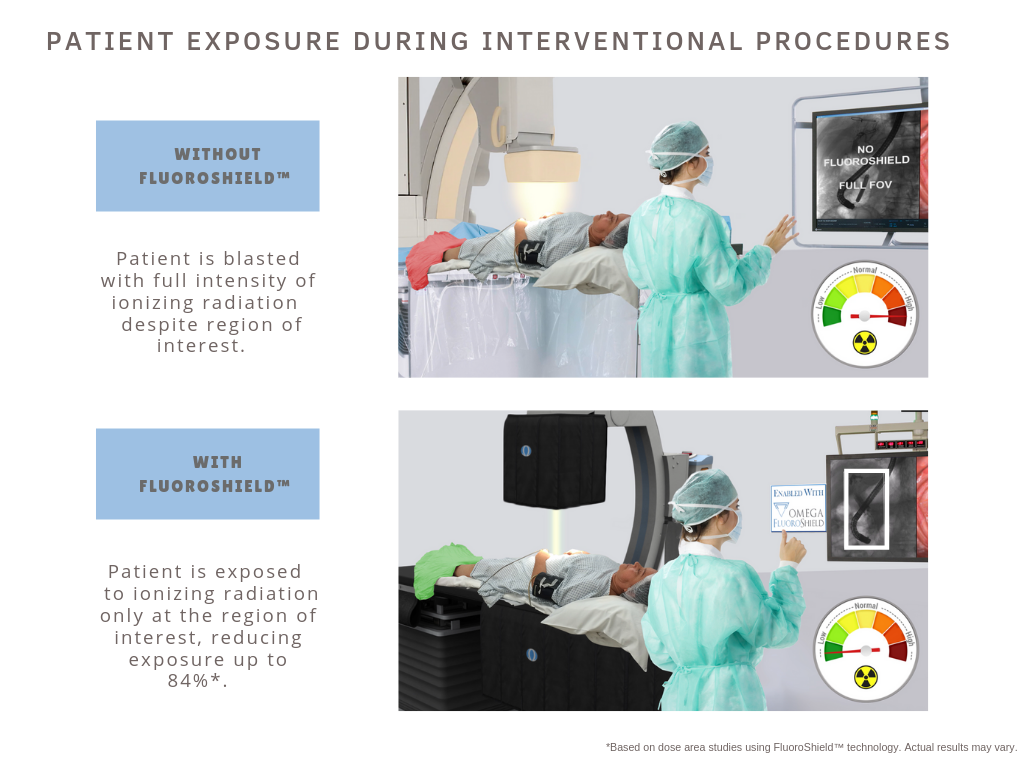
The unique FluoroShield™ system allows for auto collimation during interventional fluoro or cine cases while maintaining a perspective of surrounding anatomy. The blended image incorporates a lower frequency refresh of the peripheral image area. This combined image (live fluoroscopy or cine of ROI + background refreshed at a rate of once or twice per second) increases the quality of information presented during interventional procedures. Brian Fleming, President of Omega Medical Imaging states, “Until now products on the market have only been able to manage radiation to patients and staff. FluoroShield™ is the only system in the world that provides an actual reduction in dose. The impact of this groundbreaking solution for patients and healthcare providers is substantial. I am very grateful to be a part of a team that pushes the envelope in the development of safer healthcare solutions.”
Benefits of FluoroShield™
- Image quality is improved via auto-collimation resulting in a reduced FOV and subsequently less exposure to harmful X-ray.
- Anatomical landmarks and devices visible outside the Region of Interest (ROI) provide important clinical information which are viewed at a reduced exposure level / Rate.
- Fluoroshield™ can be sized and positioned in manual mode, as opposed to conventional collimation, which is generally limited to positioning about the center of the image.
- Auto ROI automatically follows the movement of devices i.e., catheters, etc., minimizing distraction and input requirements for the operator.
See Full Press Release: Omega Medical Imaging First in the World to Receive FDA Clearance on Artificial Intelligence Imaging System that Reduces Dose – Omega Medical Imaging
Written by: Omega Medical Imaging

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.

