Medical device and MedTech insights, news, tips and more
Orthofix Completes Acquisition of FITBONE Limb Lengthening System
March 26, 2020
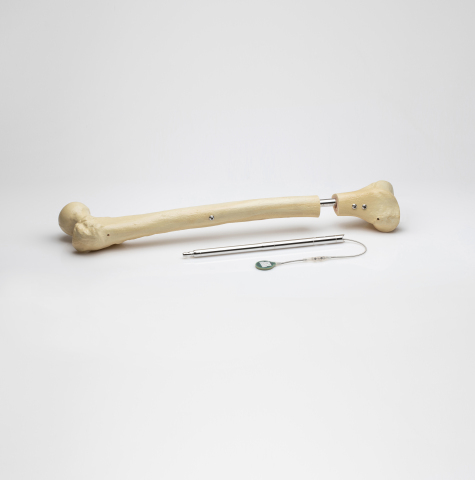
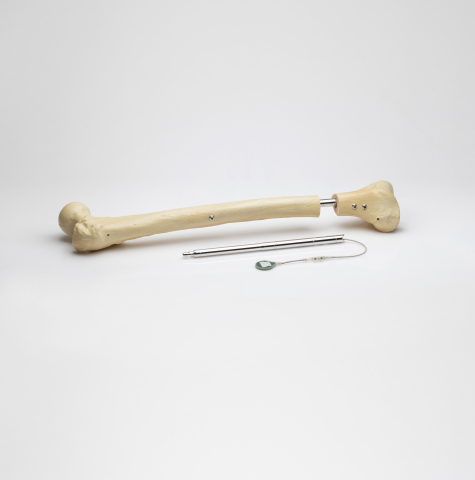
Orthofix Medical Inc., a global medical device company focused on musculoskeletal healing products, today announced it has completed the acquisition of assets associated with the FITBONE® intramedullary lengthening system for limb lengthening of the femur and tibia bones. The transaction also includes other potential applications of the technology which are in development, including the FITSPINE® system for fusionless surgery to treat early onset scoliosis. With the addition of the FITBONE assets, Orthofix becomes the only orthopedic company that offers a comprehensive portfolio of both internal and external fixation solutions for limb reconstruction.
“The acquisition of the FITBONE® intramedullary lengthening system further demonstrates Orthofix’s commitment to investing in differentiated products that are a strong strategic fit within our core businesses,” said Orthofix President and Chief Executive Officer Jon Serbousek. “Adding this technology to our current limb reconstruction portfolio enables us to offer physicians solutions to meet the needs of their patients which may require internal or external fixation procedures. We look forward to developing other applications of this exciting platform.”
Terms of the agreement executed with Wittenstein SE, a privately-held Germany-based company who developed the technology, include $18 million in cash closing consideration and a manufacturing supply contract with Wittenstein SE.
The FITBONE system will be a part of the Orthofix Extremities portfolio of solutions that include the TL-HEX™ computer-assisted ring fixation system for external limb lengthening and the eight-Plate Guided Growth System™ for correcting angular growth deformities in pediatric patients. To learn more about Orthofix’s dedication to helping surgeons and limb deformity correction patients, please visit JuniOrtho.club.
Consisting of an intramedullary lengthening nail that is surgically implanted in the bone through a minimally invasive procedure, the FITBONE system includes an external telemetry control set that manages the distraction process. The patient’s treatment is also supported through the FITBONE app that guides the patient throughout the limb-lengthening treatment. Over 3,500 cases in more than 15 countries have been performed with the FITBONE system.
The FITBONE intramedullary lengthening system is available in the U.S. under a U.S. Food and Drug Administration 510(k) clearance and in European Countries under a CE Mark approval.
See Full Press Release: Orthofix Completes Acquisition of FITBONE Limb Lengthening System | Business Wire
Written by: Orthofix

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.