Medical device and MedTech insights, news, tips and more
Phagenesis Wins FDA Breakthrough Nod for Phagenyx System for Restoring Swallowing
January 14, 2020
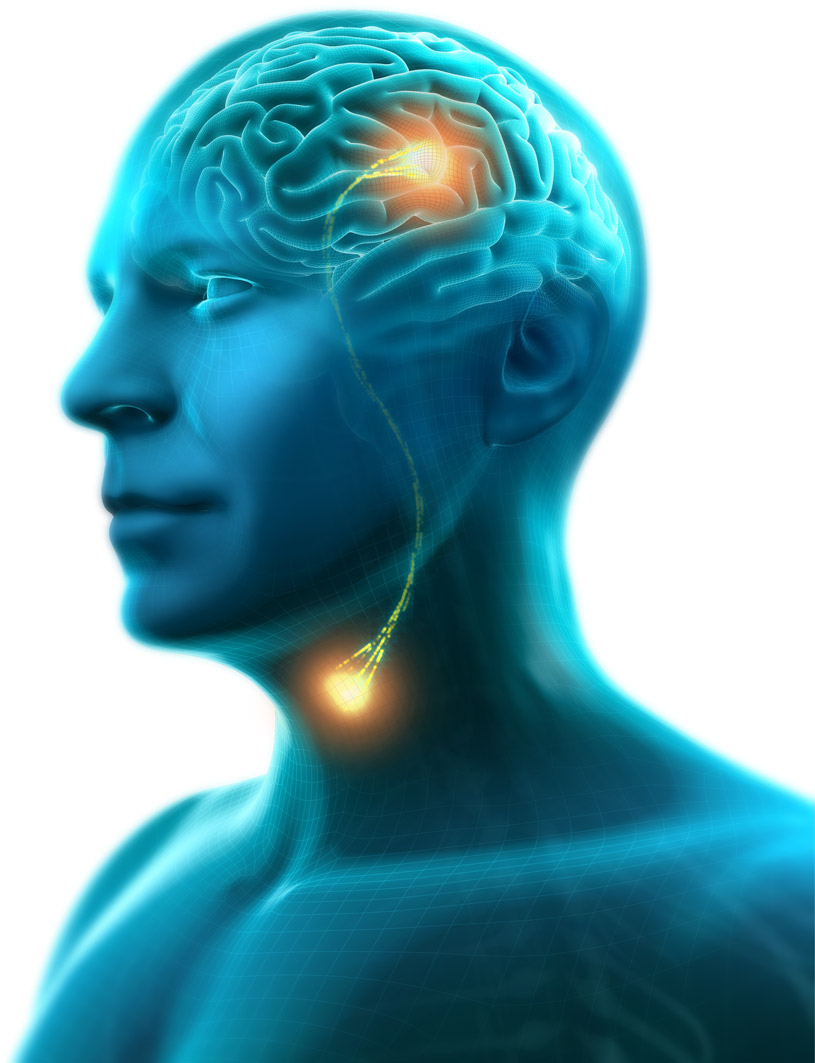
Phagenesis Ltd., a pioneering leader in the treatment of dysphagia, is pleased to announce today that the company has received Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA) for its Phagenyx System. The Phagenyx System is a novel neurostimulation device that helps to restore neurological swallowing control through Pharyngeal Electrical Stimulation.
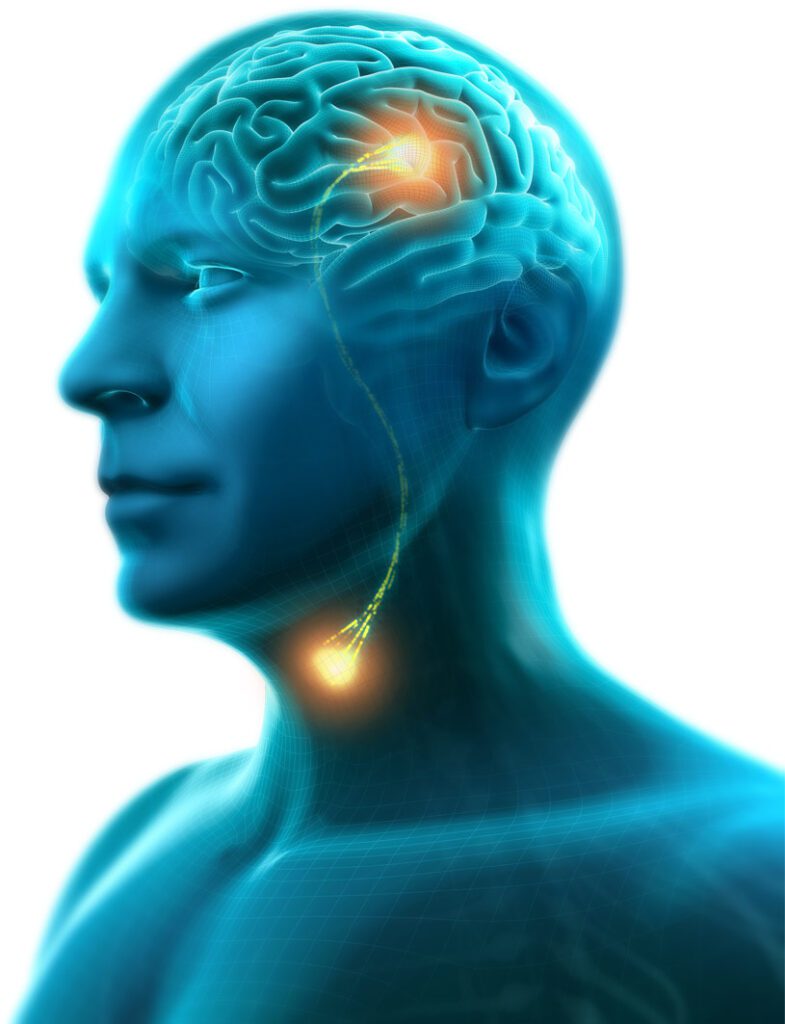
The FDA Breakthrough Device Program is intended to help patients and health care providers receive more timely access to medical devices that have the potential to provide more effective treatment or diagnosis for life-threatening or irreversibly debilitating diseases or conditions. Under the program, the FDA will provide Phagenesis with priority review and interactive communication during the Phagenyx De Novo review phase.
Reinhard Krickl, CEO of Phagenesis, said: “Receiving Breakthrough Device Designation is a key milestone, validating Phagenyx as a unique solution to accelerate decannulation that addresses a substantial unmet medical need currently causing negative consequences for risk of pneumonia. This will lead to timely and successful rehabilitation, better patient comfort and positively impact on number of days in hospital, hospital readmissions and the financial cost of care.”
The Phagenyx System treats the cause of dysphagia. In tracheotomised patients weaned from mechanical ventilation, severe dysphagia with related insufficient airway protection is the primary reason why decannulation cannot be performed. The PHAST-TRAC1 randomised controlled study, published in Lancet Neurology in 2018, demonstrated that tracheotomised patients treated with the Phagenyx System were 5 times more likely to be safely decannulated when compared with untreated control patients.
Greg Behar, CEO of Nestlé Health Science, commented: “Nestle Health Science has been working with Phagenesis for several years in Europe to build the clinical evidence for this treatment and we are excited that the Breakthrough Device Designation will help to accelerate the process of making Phagenyx available to US patients also.”
See Full Press Release: Phagenesis Receives FDA Breakthrough Device Designation for Its Phagenyx® System to Accelerate Removal of the Breathing Tube by Treating Neurogenic Dysphagia
Written by: Phagenesis Ltd.

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.
