Medical device and MedTech insights, news, tips and more
Philips Receives FDA 510(k) Clearance for Lumify S4-1 Ultrasound Transducer
October 17, 2016
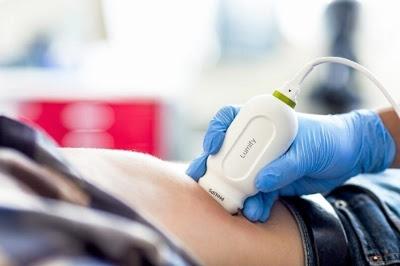
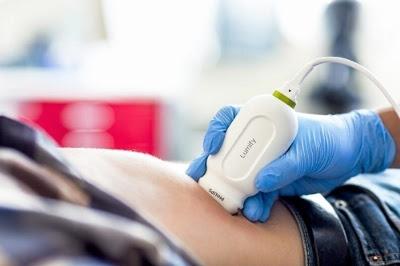 October 14, 2016 — Philips announced at The American College of Emergency Physicians’ (ACEP) annual meeting that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its S4-1 cardiac transducer for Lumify, its smart-device diagnostic ultrasound solution. The pocket-sized and lightweight S4-1 transducer now offers advanced sensitivity and high-resolution 2-D image quality, along with new exam pre-sets, allowing clinicians to quickly triage and assess their patients.
October 14, 2016 — Philips announced at The American College of Emergency Physicians’ (ACEP) annual meeting that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its S4-1 cardiac transducer for Lumify, its smart-device diagnostic ultrasound solution. The pocket-sized and lightweight S4-1 transducer now offers advanced sensitivity and high-resolution 2-D image quality, along with new exam pre-sets, allowing clinicians to quickly triage and assess their patients.
Introduced in 2015, Lumify, the Philips smart device-powered ultrasound solution, helps healthcare professionals make fast, informed decisions. Now Lumify is the first Philips ultrasound device for ambulatory use, and with the S4-1 transducer, its clinical applications are expanded to include a full offering of in-demand cardiac, abdominal including lung, OB/GYN and FAST (Focused Assessment with Sonography in Trauma) exam pre-sets. With Lumify’s full suite of point-of-care transducers, physicians in emergency care situations can take advantage of every crucial moment without the time and mobility restrictions of locating an ultrasound cart, which is often in use and/or in another department.
Watch a video explaining the Lumify system as part of the “Editor’s Choice of the Most Innovative Imaging Technology at RSNA 2015.”
“Lumify is a game-changing innovation,” said John Bailitz, M.D., emergency ultrasound physician and leader with ACEP and the Social Media and Critical Care (SMACC) organization. “The affordability, flexibility and versatility of Lumify make it appealing to those working in emergency settings, and now with the S4-1 cardiac probe and FAST exam pre-sets, we can conduct critical exams at the point of care, resulting in more efficient triage of patients.”
The Lumify app and all three transducers (L12-4, C5-2, and S4-1) completed rigorous environmental and durability testing to ensure reliability for emergency, critical care and ambulance use. The S4-1 transducer and cable weighs 152 grams and is smaller than a smartphone, adding to its versatility and mobility. Beyond integrating with everyday technology — such as off-the-shelf, compatible smart devices — Lumify also uses cloud-enabled technology to connect with picture archiving and communication systems (PACS), shared networks and system directories. Additionally, data will be accessible on the Philips HealthSuite Digital Platform, an open and secure, cloud-based IT infrastructure, allowing clinicians and health systems access to powerful data and analytics to help improve patient care.
In addition to the subscription pricing model, Lumify is also now available through a one-time purchase transaction.
Lumify will also be showcased at the Radiological Society of North America (RSNA) 2016 annual meeting, Nov. 27-Dec. 3 in Chicago.
For more information: www.philips.com
