Medical device and MedTech insights, news, tips and more
Philips Wins FDA Clearance for EMS Remote Monitoring and Defibrillation Solution
July 30, 2020
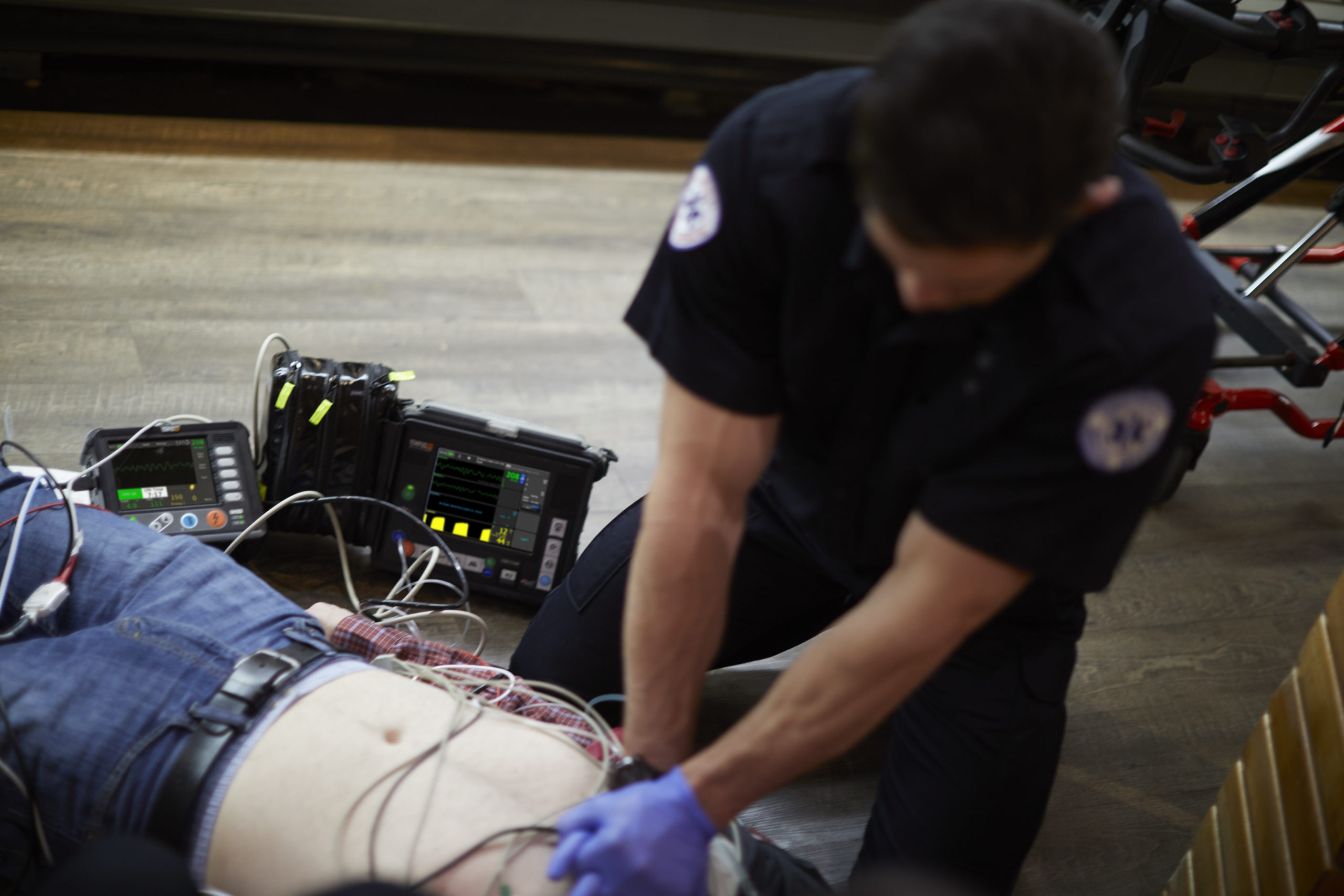
As a unique modular platform, the remote monitoring and defibrillator solution (Tempus ALS) consists of a remote portable vital signs patient monitor (Tempus Pro), and remote professional defibrillator (Tempus LS-Manual). While the monitor and defibrillator can be used separately, the devices also connect wirelessly to share data and transfer vitals, waveforms and images into Philips web-based software platform (IntelliSpace Corsium). The software platform provides robust, real-time transfer of clinical data and events, interactive ECG measurement, two-way communication and more, enabling rapid clinical and transport decision support and seamless electronic patient care recording (ePCR) integration outside the hospital in emergency settings.
 “In emergency situations, where seconds count, having access to advanced patient data collection and sharing and real-time secure data streaming, can help inform confident treatment and transport decisions outside the hospital,” said Arman Voskerchyan, General Manager of Therapeutic Care at Philips. “The integrated remote monitoring and defibrillator solution combined with our web-based software platform will help front line responders provide emergency care, diagnosis and treatment – including defibrillation therapy, data management and clinical and operational efficiency features – in a fully integrated solution.”
“In emergency situations, where seconds count, having access to advanced patient data collection and sharing and real-time secure data streaming, can help inform confident treatment and transport decisions outside the hospital,” said Arman Voskerchyan, General Manager of Therapeutic Care at Philips. “The integrated remote monitoring and defibrillator solution combined with our web-based software platform will help front line responders provide emergency care, diagnosis and treatment – including defibrillation therapy, data management and clinical and operational efficiency features – in a fully integrated solution.”
Emergencies and care events outside the hospital continue to rise, with an estimated 240 million calls made to 9-1-1 in the U.S. each year [1]. In addition to the stress of the unknown and what to expect at the scene of the call, emergency medical providers must deal with manual handling issues. Equipment carried is heavy, often damaged due to use in unpredictable conditions and has limited data connectivity – inhibiting the ability for on-scene support. In an effort to address these challenges, both elements of the Philips remote monitoring and defibrillator (Tempus ALS) solution are designed with a small, rugged exterior and long-lasting battery to allow emergency medical providers to focus on caring for the patient without the hassle or distraction of bulky equipment.
See Full Press Release at the Source: Philips Launches Tempus ALS in U.S. – News | Philips
Press Release by: Phillips
 Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.
