Medical device and MedTech insights, news, tips and more
PhotoniCare Announces FDA Clearance for First-In-Class Technology for Imaging the Ear
January 6, 2020
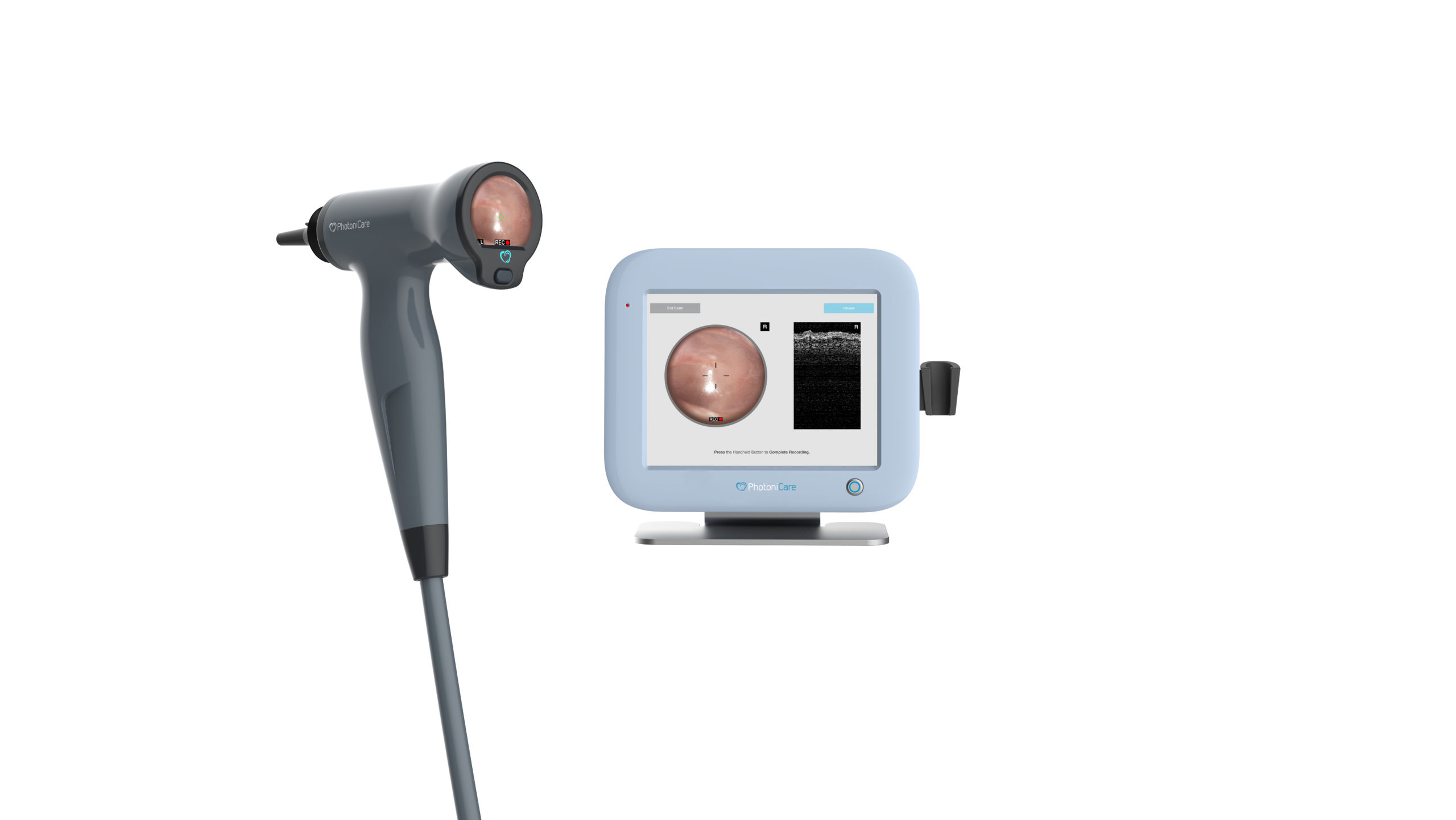
PhotoniCare, Inc., a company dedicated to revolutionizing healthcare by providing physicians with better diagnostic tools, today announced that the U.S. Food & Drug Administration (FDA) has granted 510(k) clearance for its TOMi™ Scope for non-invasive imaging of the middle ear. Using optical coherence tomography (OCT) high resolution depth imaging, TOMi Scope helps to determine the presence or absence of fluid in the middle ear and to characterize the fluid type.
According to clinical guidelines, the presence of fluid in the middle ear is a primary indicator for determining infection. Designed to eliminate subjectivity and speculation, TOMi Scope allows physicians, for the first time, to directly visualize fluid in the middle ear, where ear infections reside, and to measure the fluid’s density, even in the presence of wax – providing objective data upon which to base their decisions.
“Current tools can only provide a view of the surface of the eardrum, forcing physicians to make an assessment with very limited information, or to employ invasive surgical procedures to accurately identify middle ear pathologies,” said Diego Preciado, M.D., Ph.D. of the Children’s National Hospital in Washington, D.C., a lead investigator for clinical studies of the device. “TOMi Scope’s advanced light-based technology could dramatically alter the way children with ear problems are evaluated, enhancing our ability to inform optimal treatments.”
Middle ear infections are the leading cause of hearing loss, surgery and antibiotic use, especially in children. Affecting more than 80% of children, they are misdiagnosed up to 50% of the time using the current gold standard, examination via otoscope.1 Children can suffer from recurring ear infections for six to 12 months before they are referred to an ENT specialist, and are often prescribed increasingly potent antibiotics during this time. More than one million tube surgeries are performed in the U.S. alone every year.
“Clinicians seeking to be more accurate and confident in their assessment of middle ear fluid and fluid type will find this new technology using light wave detection very helpful,” said Michael Pichichero, M.D., Director of the Rochester General Hospital Research Institute and Research Professor at the Rochester Institute of Technology in New York. “The fact that it works well even in the presence of significant wax build-up is of great value. Wax continues to be a huge problem in middle ear diagnosis.”
“At PhotoniCare we set out to solve the massive problem of frequent misdiagnosis of middle ear infections, and the overuse of antibiotics and referrals to surgery in children that result,” added PhotoniCare co-founder & CEO Ryan Shelton. “We thank the FDA for clearing our TOMi Scope under a new product code unique to our technology, and look forward to bringing this innovation to doctors and patients very soon.”
PhotoniCare expects to immediately launch the TOMi Scope in a limited release in select U.S. geographies, with a full national launch later in 2020.
See Full Press Release: PhotoniCare Announces FDA Clearance for First-In-Class Technology for Imaging the Ear | Business Wire
Written by: PhotoniCare, Inc.

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.

