Medical device and MedTech insights, news, tips and more
Quibim Receives FDA 510(k) Clearance for qp-Prostate AI Solution for Prostate MRI Analysis
March 14, 2021
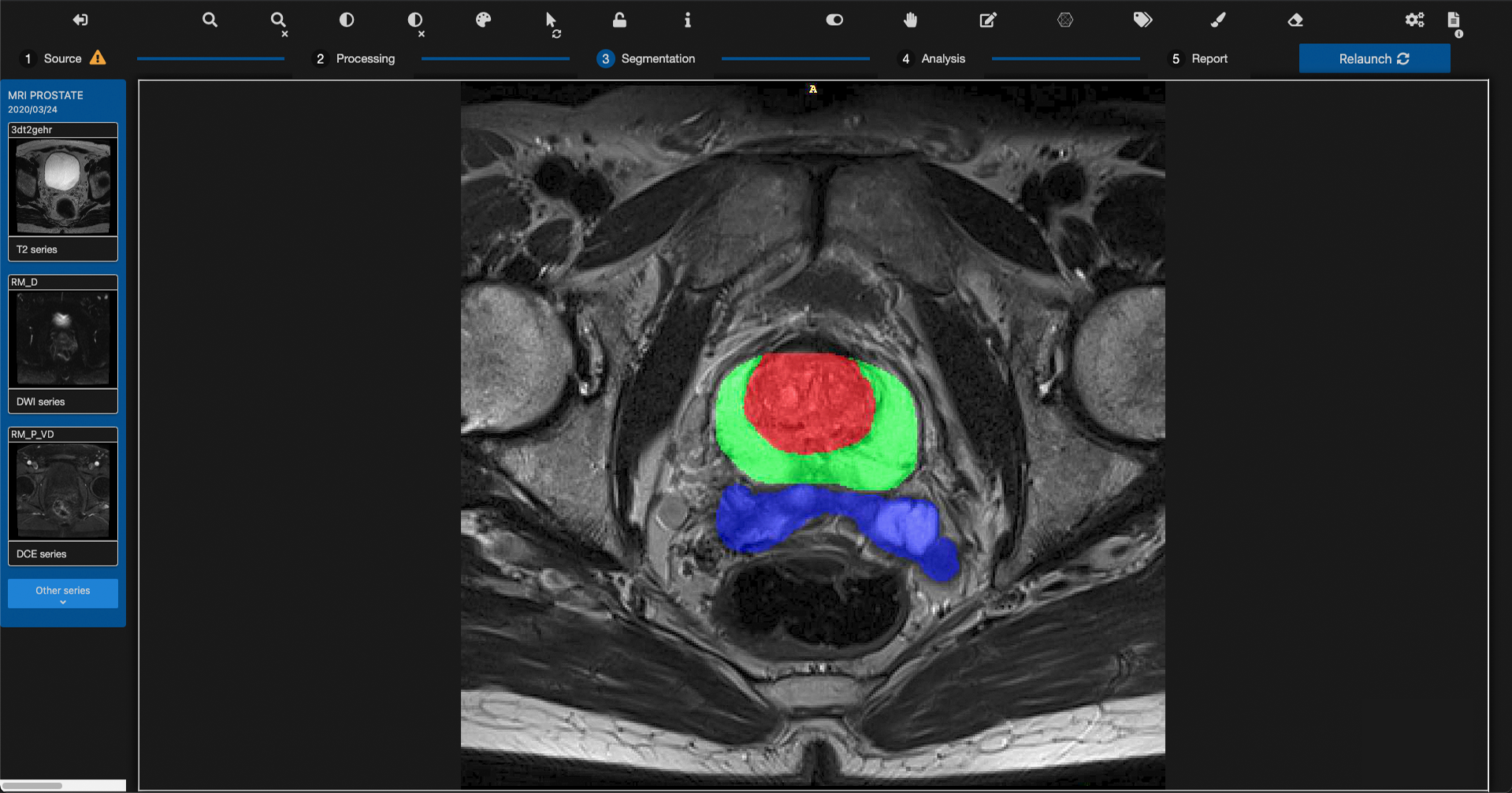
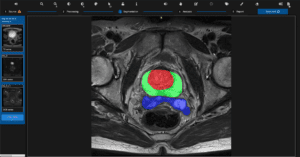
Quibim, a global leader in whole-body medical imaging analysis, announced today the launch of qp-Prostate, its latest and most advanced prostate AI based Magnetic Resonance (MR) solution, after receiving 510(k) clearance by the US Food and Drug Administration. The solution aids in the process of prostate magnetic resonance imaging (MRI) reporting from visualization to quantification with the aim of increasing diagnostic accuracy and, potentially, early prostate cancer detection, which may contribute to long term survival.
Quibim set out to transform prostate diagnosis and monitoring by developing a new non-invasive imaging tool using MRI data and advanced computer models to investigate the prostate anatomy in extreme detail. Quibim’s new qp-Prostate is the only tool on the market providing automated regional organ segmentation, a process that may reduce interpretation time and helps define diagnosis per region. “We challenged ourselves to build the world’s finest AI platform for prostate imaging, to help increase diagnostic precision as a tireless companion to the clinician and transform the men’s care journey,” said Dr. Angel Alberich Bayarri, Quibim CEO. “Instead of analyzing the prostate as a whole, the solution can segment the prostate’s transitional zone, peripheral zone and seminal vesicles, as well as other regions defined in the Prostate Imaging Reporting and Data System (PI-RADS) v2.1 guidelines to extract clinically meaningful quantitative information from the MRI examination as a potential aid for early and accurate clinicians’ diagnosis.”
This groundbreaking solution will more efficiently provide information to aid in the diagnosis of prostate cancer, the second most common cancer in men, annually affecting 1.4 million globally. With nearly 1 in 8 men diagnosed with the disease during a lifetime, early detection is important but a challenge for oncologists as early-stage prostate cancer is asymptomatic. Current diagnostic tools like measuring prostate-specific antigen (PSA) in blood have known limitations and may result in patients undergoing invasive biopsies or even unnecessary surgeries.
A new era
The past twelve months have made us question our relationship to the world. Physical distancing has challenged the way we relate to each other and we’ve all had to create new opportunities to live and work together. That’s why we’ve decided to revamp our brand identity, by focusing on the motor and the purpose of our technological innovations: the human being.
The human body is a language. Some people only speak foot, or brain, or liver. We speak human. The entire body and all of its parts. It’s a complicated system. But with our whole body medical imaging ecosystem that knows the functioning of each body part, we make it easy for clinicians and patients. Interpreting every centimetre of every body. It’s a language of care. It’s a language of discovery. And now everyone can learn it.
The website now displays photographs of clinicians at work and patients in real life, and classifies products per area to ease navigation. The logo holds on to its palette of colors but has been simplified and the company’s name has changed from QUIBIM to Quibim for a more viewer-friendly and communicative experience.
“Without renouncing to our DNA – improving health through the use of quantitative imaging biomarkers in medicine -, we want to show that AI and technology are made by humans for humans,” Alberich Bayarri said.
About Quibim
Quibim, a company with its headquarters in Valencia, Spain, is a global leader in whole-body medical imaging analysis. Quibim products are used worldwide by leading research teams. Partners use Quibim Precision®, a whole-body imaging ecosystem, for a wide range of applications from detecting a disease to tracking the efficacy of novel treatments.
Quibim follows an AI-first approach to help detect pathologies across every body part and imaging modality, using quantitative imaging biomarkers. We understand every body part. We speak human.
See Full Press Release at the Source: Quibim Receives FDA 510(k) Clearance for qp-Prostate, a New AI Solution for Prostate MRI Analysis | Quibim
Press Release by: Quibim
 Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.