Medical device and MedTech insights, news, tips and more
Rapid Medical Receives CE Mark for Stentriever
July 27, 2020
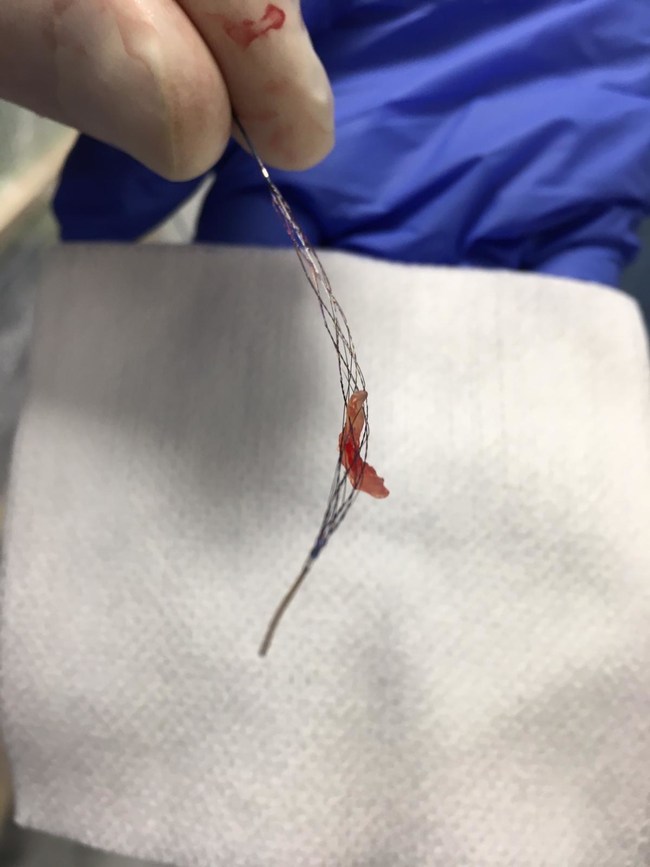
Rapid Medical, a company focused on the development of next generation neurovascular devices, has announced that it received CE Mark for TIGERTRIEVER XL. In addition, the first patients have been treated successfully with the device.
The TIGERTRIEVER family of stentrievers are the first-ever adjustable, fully visible clot retrievers designed to treat ischemic stroke. Thousands of patients have been successfully treated with TIGERTRIEVER to date. TIGERTRIEVER XL is the newest addition to the portfolio. Its adjustable diameter can conform to any vessel diameter up to 9mm and at 53mm in length, it is the largest and the longest stentriever available. Although significantly larger than other similar devices on the market, it is easily delivered via a standard microcatheter with an internal diameter of 0.021″.
“TIGERTRIEVER XL is a very important addition to the ischemic stroke market,” said Dr. Sebastian Fischer, a senior Interventional Neuroradiologist at Bochum University Hospital, Germany. “For the first time, we have a tool that can be adjusted to these dimensions and is dedicated to retrieve large clot loads. This is an important addition to our current tool set, since it can potentially reduce the number of passes required to remove large stroke-causing clots, for example, in the internal carotid artery.”
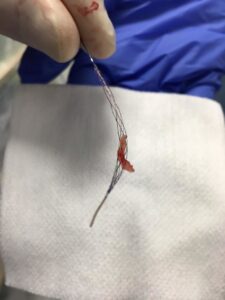
Clot removed from the brain of the 1st stroke patient treated with Tigertriever XL at Bochum University Hospital
Ronen Eckhouse, CEO of Rapid Medical commented on the first clinical experience with TIGERTRIEVER XL: “TIGERTRIEVER XL is another example of our powerful technology that adjusts to the vessel diameter for full clot removal in the treatment large vessel occlusion. TIGERTRIEVER XL compliments TIGERTRIEVER 13, the smallest stentriever available today, and the only stentriever indicated for distal vessel occlusion. The TIGERTRIEVER portfolio now allows surgeons to treat ischemic stroke in the majority of vessels where it occurs.”
See Full Press Release at the Source: Rapid Medical Receives CE Mark for TIGERTRIEVER™ XL
Press Release by: Rapid Medical
 Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.
