Medical device and MedTech insights, news, tips and more
RapidAI Receives FDA Clearance of Rapid LVO For Identification of Suspected Large Vessel Occlusions
August 13, 2020
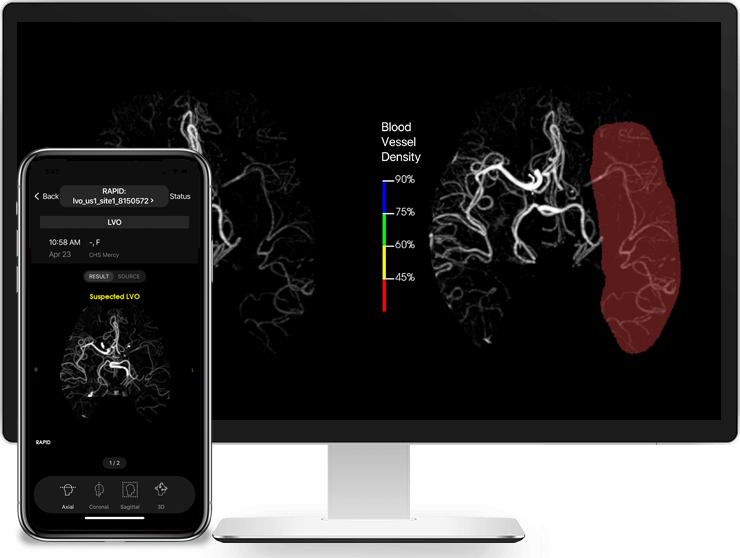
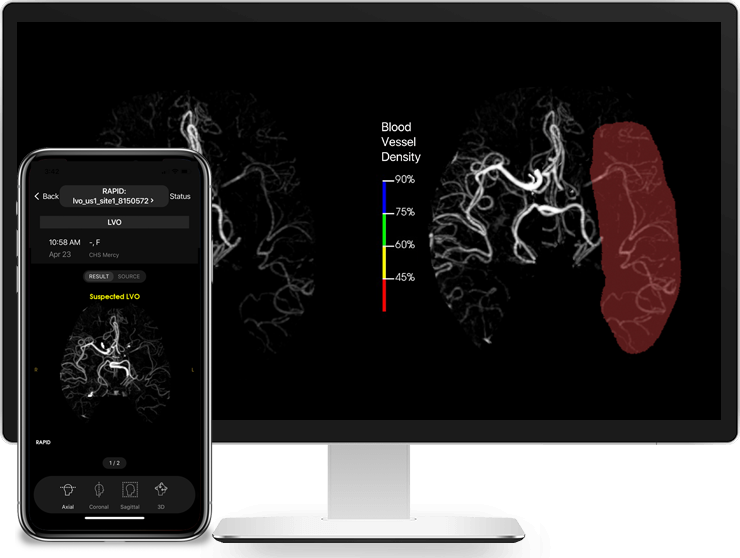
RapidAI, the worldwide leader in advanced imaging for stroke, today announced that Rapid LVO has received Food and Drug Administration (FDA) clearance for detecting suspected LVOs (Large Vessel Occlusions).
Rapid LVO helps physicians speed up triage or transfer decision-making. Working in as few as 3 minutes, Rapid LVO uses a vessel tracker in conjunction with assessment of brain regions with reduced blood vessel density to identify suspected LVOs with a sensitivity of 97% and a specificity of 96%. Stroke team members are also immediately notified when a suspected LVO is detected.
“LVOs are the most disabling and deadly ischemic strokes,” said Dr. Greg Albers, Professor of Neurology at Stanford University, Director of the Stanford Stroke Center and cofounder of RapidAI. “The ability to identify LVOs rapidly facilitates more effective treatment. This is why we are very excited about the FDA clearance of Rapid LVO, a significant step forward in stroke diagnostics and care.”
RapidAI makes the most-widely used stroke imaging software for patient care, research, and clinical trials—helping hospitals around the world save time, money, and lives. Rapid® is the only clinically validated platform available and considered by many to be the gold standard for advanced cerebrovascular imaging.
RapidAI is the worldwide leader in advanced imaging for stroke. Based on intelligence gained over 1,000,000 scans from more than 1,600 hospitals in over 50 countries, the Rapid® platform uses artificial intelligence to create high quality, advanced images from non-contrast CT, CT angiography, CT perfusion, and MRI diffusion and perfusion scans. The Rapid imaging platform includes Rapid ICH, Rapid ASPECTS, Rapid CTA, Rapid LVO, Rapid CTP, and Rapid MRI. RapidAI also offers SurgicalPreview®, a comprehensive aneurysm management platform.
See Full Press Release at the Source: RapidAI Receives FDA Clearance of Rapid LVO For Identification of Suspected Large Vessel Occlusions | Business Wire
Press Release by: RapidAI
 Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.