Medical device and MedTech insights, news, tips and more
RapidAI Receives First and Only FDA 510(k) Clearance of Non-Contrast CT Imaging Product to Accelerate Acute Stroke Triage
April 19, 2023
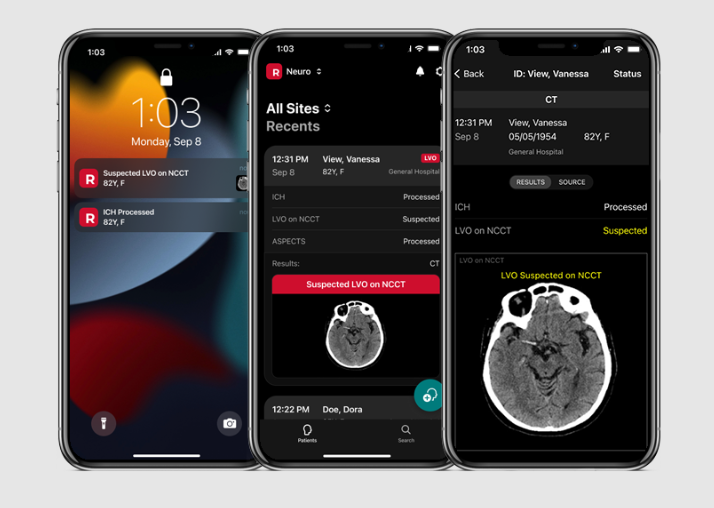
RapidAI, the global leader in neurovascular and vascular AI-enhanced clinical decision support and patient workflow, today announced it has received FDA 510(k) clearance for Rapid NCCT Stroke – a major addition to the RapidAI suite of non-contrast based solutions for stroke and trauma care, and the first and only FDA cleared medical device to detect suspected intracranial hemorrhage (ICH) and large vessel occlusion (LVO) from value-based CT imaging.
Rapid NCCT Stroke uses artificial intelligence to analyze non-contrast CT (NCCT) images to determine suspicion of ICH and LVO of the distal internal carotid artery (ICA) and middle cerebral artery (MCA-M1). The fully automated system then delivers triage and prioritization notifications through PACS, email, and the Rapid mobile app. Because NCCT imaging is readily available and is the initial imaging modality used for stroke and trauma patients, care teams can make time-sensitive workflow and transfer decisions faster. For hospitals that do advanced imaging, Rapid NCCT Stroke may significantly reduce the time between CT and CTA scans.
Rapid NCCT Stroke supports teams in:
- Accelerating door-to-imaging and door-to-decision time
- Making faster transfer decisions
- Improving equity of care by giving centers of all levels greater access to advanced value-based CT imaging technology that may improve decision making and impact patient outcomes
“This technology will not only have an enormous impact on stroke care here in the U.S. but also globally, by giving care teams at small, local, or regional facilities around the world access to advanced clinical decision support technology too often only available at comprehensive stroke centers,” said Karim Karti, CEO of RapidAI. “Our hope is that by providing better information early for more informed treatment and transfer decisions, Rapid NCCT Stroke will support faster stroke care and better patient outcomes. This is an incredible achievement for the team and yet another example of RapidAI’s continued leadership in creating the next evolution of stroke care technology.”
To learn more about how RapidAI is transforming stroke care, please visit: https://www.rapidai.com/stroke
About RapidAI
RapidAI is the global leader in using AI to combat life-threatening vascular and neurovascular conditions. Leading the next evolution of clinical decision-making and patient workflow, RapidAI is empowering physicians to make faster decisions for better patient outcomes. Based on intelligence gained from nearly 10 million scans in more than 2,000 hospitals in over 100 countries, the Rapid® platform transforms care coordination, offering care teams a level of patient visibility never before possible. RapidAI – where AI meets patient care.
See Full Press Release at the Source: RapidAI Receives First and Only FDA 510(k) Clearance of Non-Contrast CT Imaging Product to Accelerate Acute Stroke Triage
Press Release by: RapidAI
Legacy MedSearch has more than 35 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 17 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.

