Medical device and MedTech insights, news, tips and more
SherpaPak and SherpaPerfusion Donor Heart Transport Coolers Cleared in Europe
February 22, 2018
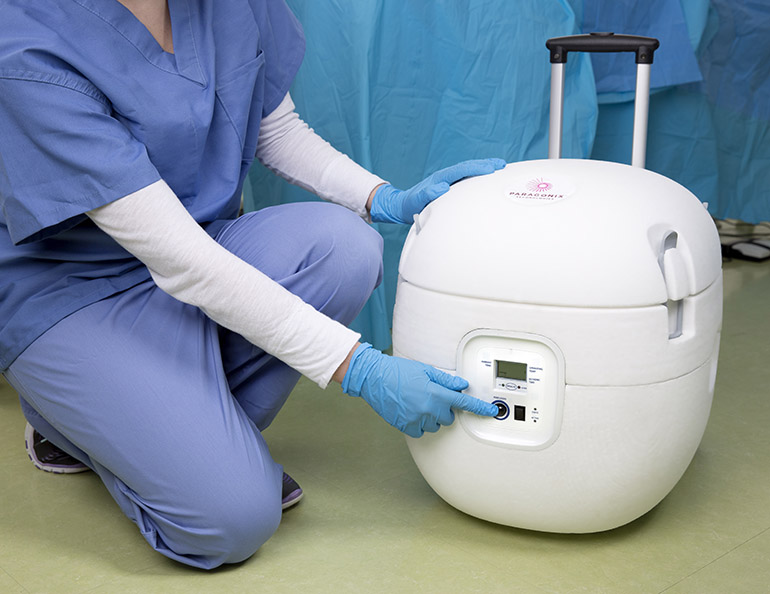
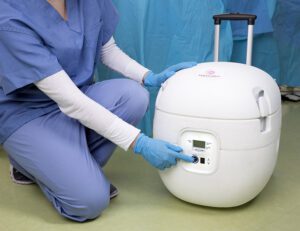
Paragonix Technologies, based in Braintree, Massachusetts, won European approval to introduce its SherpaPak and SherpaPerfusion cardiac transport systems. The devices are intended for maintaining hearts while they’re being transported from harvest in preparation for transplantation.
The SherpaPak device relies on hypothermic static preservation to transport donor hearts, while the SherpaPerfusion uses hypothermic oxygenated perfusion preservation for the same task. Both systems are single-use and disposable.
The company hopes to replace the use of conventional coolers, essentially the same that one takes to soccer and baseball games, with its SherpaPak that provides better temperature management compared to a bed of ice, while monitoring the temperature inside the chest.
Dr. Steven Tsui, Director of Transplantation & Mechanical Circulatory Support and Consultant Surgeon at the Royal Papworth Hospital, UK, in a published statement said “I am excited about the European availability of Paragonix’s two innovative donor heart preservation devices. I believe that the SherpaPak™ Cardiac Transport System addresses the shortcomings of current cold storage by improving temperature maintenance and monitoring of the donor heart during transport. As a heart transplant surgeon with over a decade’s experience of ex-vivo donor heart perfusion, I also welcome the addition of SherpaPerfusion™ Cardiac Transport System to the clinical community, utilizing oxygenated hypothermic perfusion for improved donor heart quality. I look forward to both products entering the clinic.”
Check out the Video and Read More at the Source: SherpaPak and SherpaPerfusion Donor Heart Transport Coolers Cleared in Europe | Medgadget
Photo Credit: Bryce Vickmark. www.vickmark.com 617.448.6758