Medical device and MedTech insights, news, tips and more
SMART Medical Systems Receives FDA Clearance for Its G-EYE Colonoscope
May 26, 2020
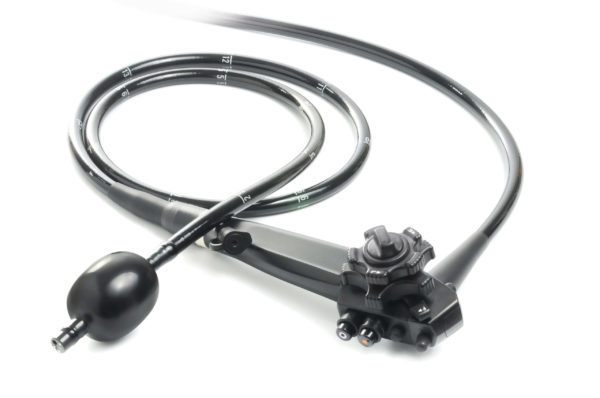
SMART Medical Systems Ltd., a developer and manufacturer of innovative endoscopy products, announced that the FDA issued 510(k) clearance for its flagship product, the G-EYE® Colonoscope.
The G-EYE® colonoscope is a standard colonoscope which SMART remanufactures by installing its proprietary G-EYE® balloon on the distal bending section of the colonoscope. During colonoscopy, the G-EYE® colonoscope is inserted in the standard technique, with the balloon deflated. Once the colon is intubated and prior to withdrawal, the balloon is inflated to engage the colon lumen. Withdrawal of the G-EYE® Colonoscope through the colon with the balloon moderately inflated centralizes the image of the colon lumen, flattens colonic folds, and reduces the amount of bowel slippage, thereby assisting in controlling the colonoscope’s field of view and positioning. In published clinical studies comparing adenoma detection rate of G-EYE® colonoscopy to that of standard colonoscopy, G-EYE® colonoscopy demonstrated substantial increase in the detection of cancerous polyps which are the precursors of colon cancer (GIE 2019; 89: 545-553; and Endoscopy 2015; 47: 238–244).
Under the current FDA clearance, the G-EYE® colonoscope will be available based on selected 510(k) cleared colonoscopes of OLYMPUS (8 models) and PENTAX Medical (3 models). SMART is currently preparing its 510(k) submission for the FUJIFILM brand.
“We are excited to have the G-EYE® colonoscope available for American patients, doctors and endoscopy practices, and are proud of taking part in the global effort to provide advanced technologies for colonoscopy”, said Brian Cochrane, CCO of SMART’s US subsidiary. “We are now initiating our launch of the G-EYE® colonoscope in the US market, aiming to make it available to clinical collaborators and customers”.
“This FDA clearance is a cornerstone for our company”, said Gadi Terliuc, SMART’s CEO. “This important milestone, adding to our recently-formed strategic partnership with FUJIFILM, is part of our evolution as a meaningful provider of enabling endoscopy products”.
See Full Press Release: SMART Medical Systems Receives FDA Clearance for Its G-EYE® Colonoscope
Written by: SMART Medical Systems

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.

