Medical device and MedTech insights, news, tips and more
Spectrum Solutions Saliva Collection Device Answers Critical Testing Hurdles, Enabling Large-Scale COVID-19 Testing While Protecting Health Professionals From Exposure
April 6, 2020
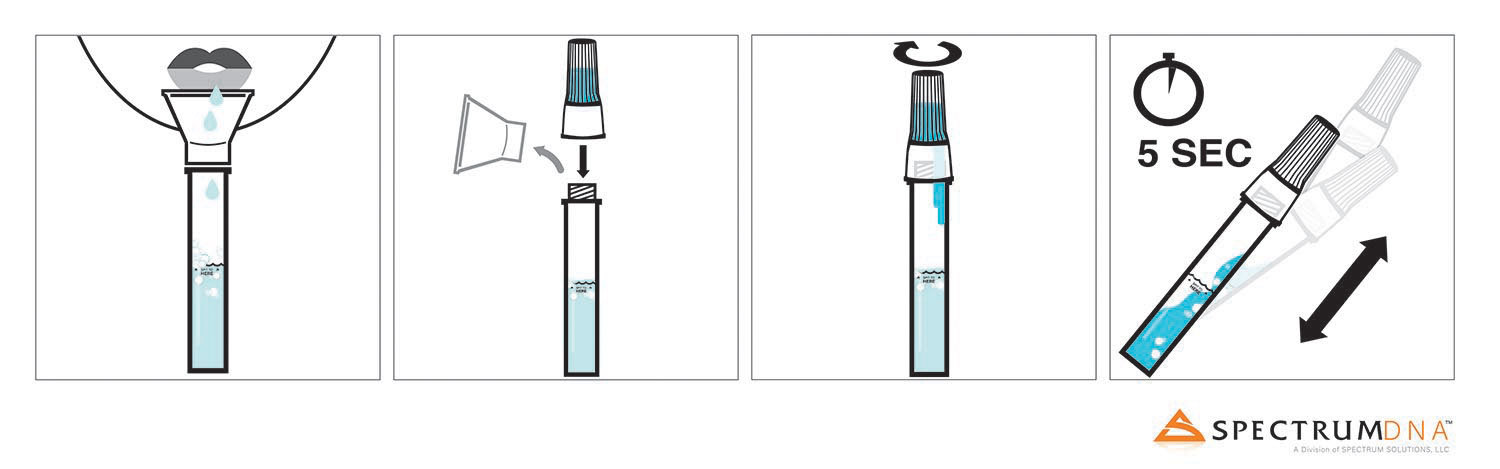
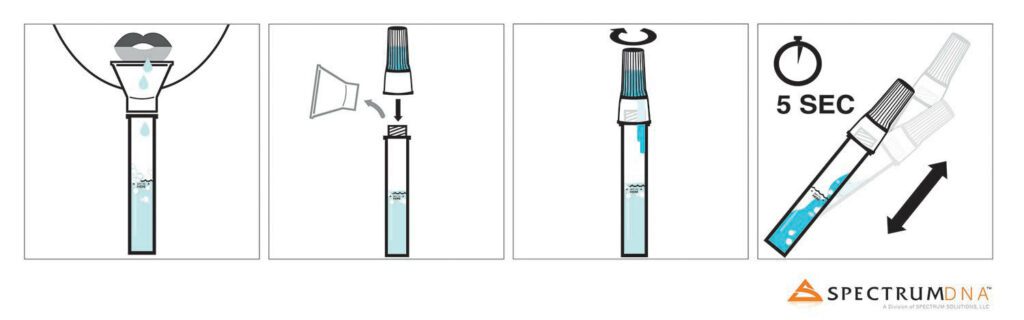
Spectrum DNA™, a division of Spectrum Solutions, LLC, in Salt Lake City, Utah, today announced that using the Spectrum DNA SDNA-1000 Whole Saliva Collection Device, researchers from RUCDR Infinite Biologics at Rutgers University have successfully validated saliva as being a viable biosample source for COVID-19 detection when compared to nasopharyngeal or oropharyngeal swabs. The resourceful discovery has come just in time to answer the consistent exponential increase in test demand.
“This is it. This is what makes America so great,” Said Bill Phillips, Spectrum’s Chief Operating Officer. “Challenge and adversity throw up roadblocks and we partner together to discover, and deliver, not only an answer to the supply shortage for sample collection but uncover a solution that is actually better than what we were using in the first place.”
Saliva testing is a new development that under a medical provider’s direction, allows for simple self-administered sample collection that even those in quarantine or self-isolation can use. Researchers at Rutgers’ RUCDR Infinite Biologics compared swab-collected biosamples head-to-head against saliva biosamples using the Spectrum DNA whole saliva collection device and its patented blue preservation solution. Saliva not only demonstrated to be a robust source of viral RNA, but when preserved with Spectrum’s patented preservation solution it was seen to protect COVID-19 transcripts for an extended period of time, making sample collection and transport to CLIA testing labs more efficient and amenable to global testing. Adding in the capability for self-collection uncovers additional opportunity to protect medical teams, helping to reduce the risk of further exposure and spread, opposed to nasal swab biosample collection requiring up-close medical assistance.
“It had been previously reported on how saliva was being successfully used for viral DNA/RNA extraction. Due to this state of emergency and medical supply shortage we decided to test it for ourselves using Spectrum saliva collection devices. Our side-by-side test comparison of over 75 patient samples demonstrated that the use of saliva to extract viral RNA was in fact a robust source for COVID-19 detection and equal in performance to the approved swab-based collection samples,” said Andrew Brooks, PhD, COO, RUCDR Infinite Biologics and Professor of Genetics at Rutgers University. “In addition, the preservation solution from the Spectrum whole saliva collection device preserved the COVID-19 viral RNA for testing, enabling for easier logistics from sample collection to the testing laboratory.”
“As demand for COVID-19 testing continues to dramatically climb, supplies for many of the critical components are just not keeping pace. The discovery that saliva is a viable and more convenient source of biomaterial for testing will immediately help hospital networks and labs, bypassing the critical shortage of supplies, including nasal and throat swabs, for COVID-19 testing,” said Stephen J. Fanning, CEO of Spectrum Solutions. “A saliva test will also allow for a broad and more expansive population screening for the virus than what has been possible to date during this global health crisis.”
“These exciting results have a tremendous impact on testing. We encourage any clinical laboratory or reference laboratory wanting to validate our protocols and submit for their EUA testing approval to contact Spectrum for guidance. The more of us approved for testing, the faster we can deliver testing relief to those desperately waiting for answers,” said Dr. Brooks.
See Full Press Release: Spectrum Solutions™ Saliva Collection Device Answers Critical Testing Hurdles, Enabling Large-Scale COVID-19 Testing While Protecting Health Professionals From Exposure | Business Wire
Written by: SPECTRUM DNA

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.