Medical device and MedTech insights, news, tips and more
Spino Modulation Vertebral Body Tethering Device Wins FDA Breakthrough Device Designation
June 20, 2021
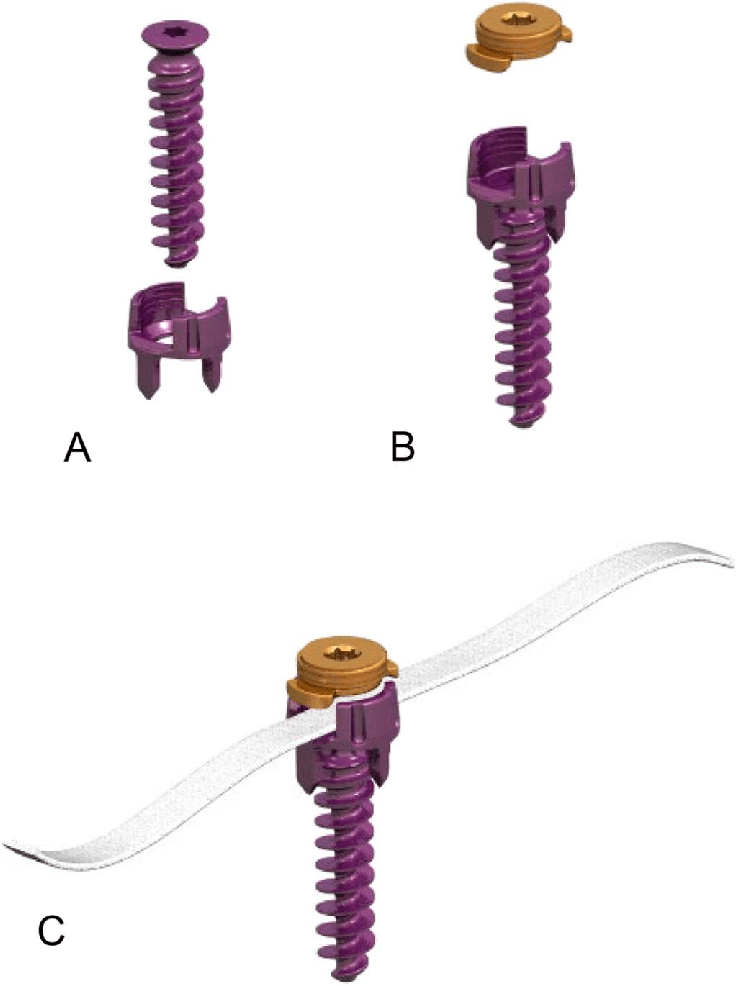
Spino Modulation Inc., a subsidiary of Spinologics Inc., announced today that the U.S. Food and Drug Administration (FDA) has granted the company a Breakthrough Device Designation for its MIScoli™ system, an innovative vertebral body tethering (VBT) device to treat scoliosis in young adolescents.
The FDA Breakthrough Device Program is intended to help patients receive more timely access to certain medical devices that have the potential to provide more effective treatment for life-threatening or irreversibly debilitating diseases or conditions, including a prioritized review of market approval regulatory submissions.
The MIScoli™ system is one of a few VBT devices that are under investigation, or limited market release, in many countries. Various medical device developers have committed to developing VBT solutions to serve an unmet need in the scoliosis surgical treatment market.
VBT is a promising and lesser invasive alternative to spine fusion, today’s standard of care when adolescents need a surgical intervention to correct a curvature of the spine. Patients, and their parents, are looking for treatment options that are less traumatic than fusion, which is associated with long in hospital and at-home recovery times, growth and mobility restrictions, longer term pain and narcotics requirements and significant surgical scars.
Adolescent Idiopathic Scoliosis (AIS) is a common and under diagnosed condition amongst adolescents across the world. It is estimated that 2-3% of the population possess some degree of AIS. If detected, physical rehabilitation and braces are used to delay the progression of spine curvature. In the more severe cases, surgical intervention is needed. Spine fusion is mostly recommended once the patient has finished growing, therefore teenagers must live with a worsening condition until early adulthood. VBT has the potential to disrupt treatment paradigms as it can be performed earlier in the patient’s history. A key mechanism to the success of this therapy is by leveraging the patient’s growing spine, known as growth modulation.
Spinologics was founded in 2010 by three Orthopedics spine surgeons from Montreal (Canada) to create an environment where they develop surgical tools for back surgery.
Spino-Modulation focuses on the development of the MIScoli™ system, including sponsoring a pivotal clinical trial in Canada.
See Full Press Release at the Source: The Food and Drug Administration (FDA) grants a « Breakthrough Device Designation » to the MIScoli™, an innovative vertebral body tethering device to treat idiopathic scoliosis
Press Release by Spino Modulation (Spineologics)
 Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.
