Medical device and MedTech insights, news, tips and more
THINK Surgical Receives FDA Clearance of Second-Generation Active Robot
November 23, 2020
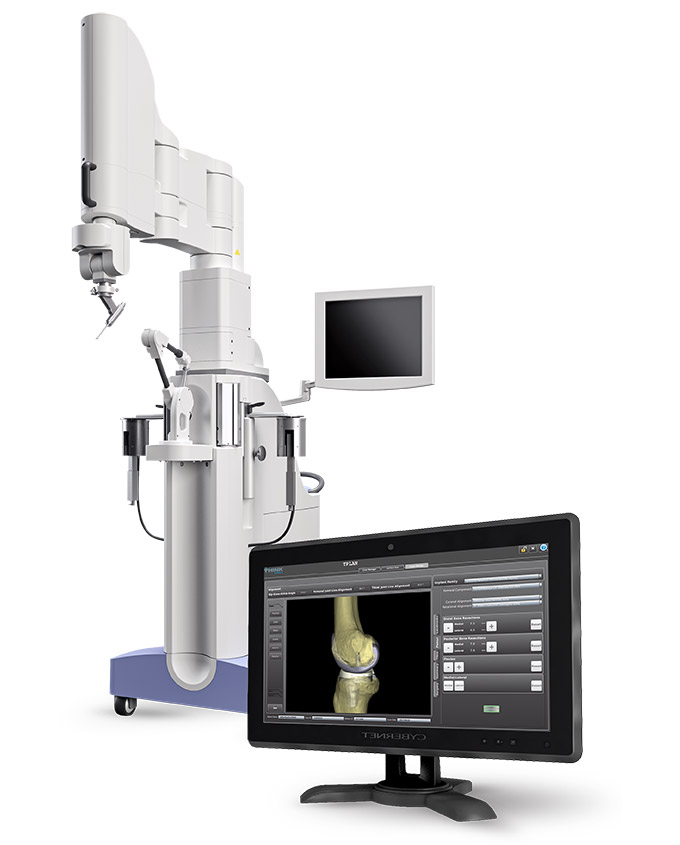
THINK Surgical, an innovator in the field of orthopedic active robot surgery, announced today that the Food and Drug Administration (FDA) has cleared the second-generation of the TSolution One Total Knee Application. The system features an active robot for total knee replacement, providing fully automated bone preparation, and gives surgeons a choice of implant options. The new system features upgrades to the current system, including an enhanced pre-surgical planning user interface, quick-change tooling, improved surgeon accessories, and advanced bone model generation. The clearance of the second-generation TSolution One Total Knee Application comes approximately one year after THINK’s first-generation active robot was cleared.
“The ongoing evolution of the TSolution One Total Knee Application is a testament to THINK Surgical’s dedication and investment in advancing the use of robot technology in the orthopedic setting,” said Jay Yang, acting CEO, THINK Surgical. “The versatile, open platform provides surgeons with the flexibility of using a variety of implants, while offering hospitals and ambulatory surgery centers a sustainable, high throughput system for their ever-increasing total knee replacement procedures.”
The TSolution One total Knee Application is comprised of TPLAN®, the 3D pre-surgical planning workstation, and TCAT®, the active robot that helps the surgeon execute each patient’s individual preoperative plan with consistent results through fully automated bone preparation. This second-generation robot incorporates several changes to the hardware and software, which significantly enhance the system’s efficiency, flexibility and ease of use while maintaining the precision and innovation that is the hallmark of the TSolution One Total Knee Application system.
About THINK Surgical, Inc.
THINK Surgical, Inc. is committed to the future of orthopedic surgery and to improving patient care through the development of leading-edge precision technology. THINK Surgical, Inc. develops, manufactures, and markets active robotics for hip and knee replacement surgery and maintains an open implant library, allowing surgeons maximum choice for their patients. For more information, please visit www.thinksurgical.com.
See Full Press Release at the Source: THINK Surgical® Receives FDA Clearance of Second-Generation Active Robot
Press Release by: Think Surgical
 Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.
