Medical device and MedTech insights, news, tips and more
TransEnterix Receives FDA Clearance for First Machine Vision System in Robotic Surgery
March 19, 2020
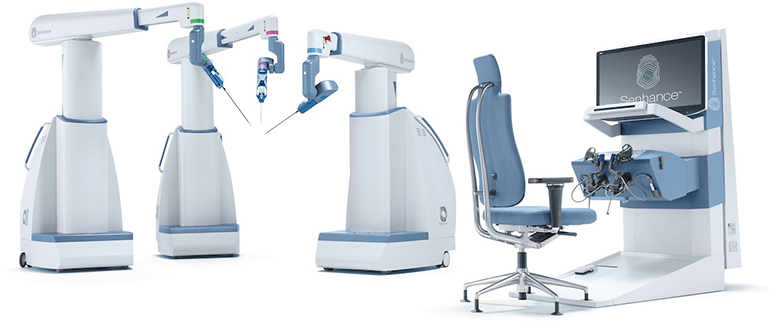
TransEnterix, Inc., a medical device company that is digitizing the interface between the surgeon and the patient to improve minimally invasive surgery, today announced the Company received 510(k) clearance for the Intelligent Surgical Unit (ISUTM) that enables machine vision capabilities on the SenhanceⓇ Surgical System.
“We are pleased to have received this important clearance earlier than expected. Machine vision is the next major advance in digital surgery,” said Anthony Fernando, TransEnterix president and CEO. “Our system is designed to significantly advance the sensing capabilities of computer-assisted surgery. With this hardware and software system, the Senhance System will gather and interpret visual information from the surgical field. The capabilities now cleared will be focused on optimizing visualization and camera control in ways never before offered in robotic or digital surgery. These initial capabilities represent the first step in our journey to bring the benefits of augmented intelligence and machine vision to surgery.”
The ISU enables machine vision driven control of the camera for a surgeon by responding to commands and recognizing certain objects and locations in the surgical field. The ISU hardware is also designed to be compatible with planned future augmented intelligence features such as scene cognition and surgical image analytics that are expected to continue to drive meaningful innovations in digital laparoscopy with Senhance.
“This is the beginning of a new era in digital surgery,” said Dr. Amit Trivedi, chair of surgery at Hackensack Meridian Health Pascack Valley Medical Center and a participant in the design and usability studies conducted in support of the 510(k) submission of the ISU. “Surgery is the skilled real time application of vision, experience, precise motion and decision making. The opportunity to use a computer to see aspects of the field and guide surgery is enormous. I am eager to utilize machine vision to better control the camera seamlessly during my surgeries.”
See Full Press Release: TransEnterix Receives FDA Clearance for First Machine Vision System in Robotic Surgery
Written by: TransEnterix

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.