Medical device and MedTech insights, news, tips and more
TransEnterix Senhance Surgical Robot Used in Radical Hysterectomy
October 19, 2016
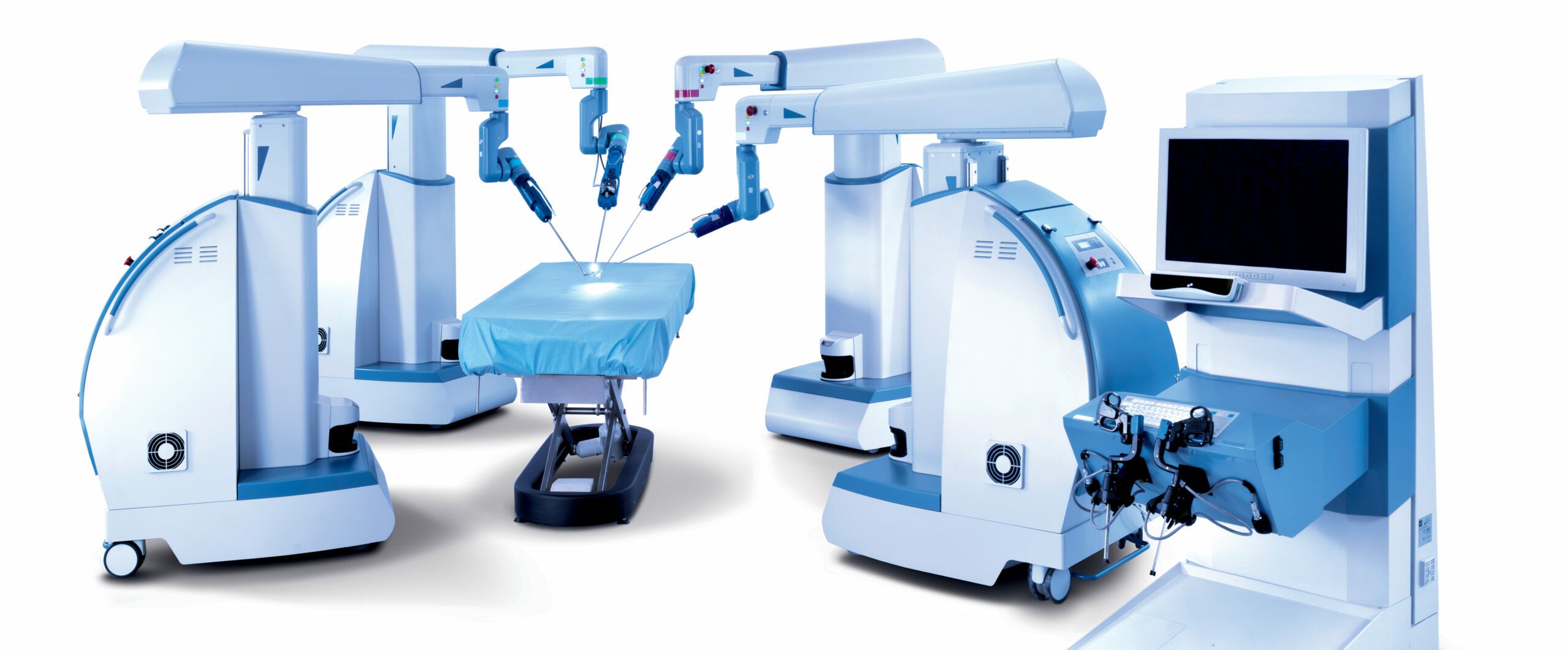
 Transenterix Inc.’s Senhance recently scrubbed in, so to speak, to lend its robotic hands to a gynecologic surgeon in Italy during a major cervical cancer procedure. It was the first time the company’s recently re-branded surgical robotic system has been used during a radical hysterectomy and, if the surgeon’s feedback is any indication, it probably won’t be the last time the Senhance is involved with a complex gynecologic oncology case.
Transenterix Inc.’s Senhance recently scrubbed in, so to speak, to lend its robotic hands to a gynecologic surgeon in Italy during a major cervical cancer procedure. It was the first time the company’s recently re-branded surgical robotic system has been used during a radical hysterectomy and, if the surgeon’s feedback is any indication, it probably won’t be the last time the Senhance is involved with a complex gynecologic oncology case.
A radical hysterectomy involves an extensive pelvic lymph node dissection and removal of the patient’s uterus and parametrium, ovaries, fallopian tubes, and part of the vagina. Salvatore Alletti, of the Policlinico A. Gemelli Foundation in Rome, said the Senhance is a promising technology for this type of procedure, because it is designed to enhance the surgeon’s precision, camera control, and ergonomics – along with providing the haptic feedback that sets Transenterix apart in the surgical robotics space.
Until about a month ago, the multi-port surgical system was known as the Alf-x, but Transenterix CEO Todd Pope told Medical Device Daily the company re-branded the robot, primarily based on surgeon feedback. After using the system for the first time, surgeons often tell the company that it “enhances their senses.”
One of the biggest selling points of the system, Pope said, is that it gives surgeons the sense of touch that they don’t get with other robotic surgical technology on the market. “That’s such an important feature for a surgeon to have, to be able to feel,” he said.
Enhancing the surgeon’s performance, as opposed to trying to replace the surgeon, has been a key focus for the company throughout the development process of the system.
“We really feel like this is a great meld of man and machine,” Pope said.
Another way the Senhance is designed to enhance the surgeon’s senses during procedures is through a 3-D camera and eye-tracking feature that allows the surgeon to move the camera with their eyes instead of having to rely on someone else to drive the camera or having to disengage from the operating arms in order to reposition the camera for a better view, as is the case both in traditional laparoscopic procedures and current surgical robotics, Pope said. The system is designed to track the surgeon’s eye movements so that when they move their eyes from top to bottom or left to right, the camera follows their gaze. The feature not only adds convenience but also saves procedure time, Pope said.
The Senhance has a fairly broad CE mark, allowing the robot to be used for general surgery as well as procedures in gynecology, urology and thoracic surgery. The company has received a lot of interest from gynecologic surgeons in particular, Pope said. He said traditionally, surgeons in Europe have been slow to adopt robotic technology because many of the procedures they do are not complex enough to qualify for the higher level of reimbursement they needed in order to justify the investment. In Europe, most of the robotics procedures that are done are prostate procedures, he said.
But Pope said the Senhance system has been developed at a lower cost compared to competing systems, so surgeons can perform even minor procedures with the technology at a lower reimbursement level and it’s still cost effective. Now, gynecologic surgeons in Europe have used the Senhance for everything from benign ovarian procedures and benign hysterectomies to the more complicated radical hysterectomies like the one Alletti performed with the system. The system is designed to work within the hospital’s ecosystem, he said, by not requiring a lot of additional capital investment, such as specialized beds or trocars.
Transenterix has had a challenging year, including an FDA rejection of its Surgibot robot and an $80.1 million net loss in the second quarter, but Pope said the company is still working with the FDA to provide clarity on the Surgibot and is “encouraged” so far by the progress on that front. He said he plans to update investors on that progress during the company’s next quarterly earnings call. The agency rejected the company’s original submission for the Surgibot because it did not meet the criteria for substantial equivalence based on the data Transenterix provided. (SeeMedical Device Daily , Aug. 8, 2016.)
Author: Amanda Pedersen
