Medical device and MedTech insights, news, tips and more
TransEnterix’s Senhance Surgical Robot Nearing FDA 510(k) Submission
March 9, 2017
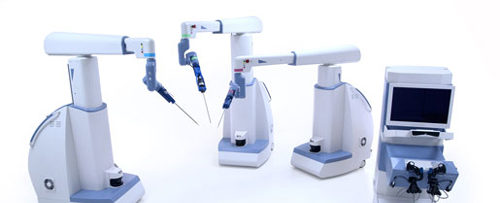
After some slight delays over the past year, TransEnterix’s Senhance System is scheduled for an FDA 510(k) submission in the next couple months, with potential clearance expected this year. The company has also been commercializing the robotic surgery system in Europe—will the go-to-market experience there inform the system’s future in the United States?
The Senhance System is slated for FDA 510(k) submission before TransEnterix’s next earnings call in early May. Management expects this timeline to lead to anticipated clearance in 2017. CEO Todd Pope told analysts on the March 6 earnings call that both usability and validation studies have been performed, reducing the risk of any delays to the submission timeline.
“You have to keep in mind now . . . we are doing surgery safely and effectively almost every day of the week and we have interacted with the FDA extensively since last summer in our presubmissions . . .” Pope said on the earnings call, according to a Seeking Alpha transcript. “We look at it that we successfully completed all of our high and medium risk elements as far as all of our testing requirements and we feel very confident that we are not only going to file before that May call but receive approval before the end of the year.”
There are now eight surgeons using the Senhance System at four different hospitals, Pope noted, and traditional laparoscopic surgeries are being performed robotically without cost concerns. “It has been encouraging to see surgeons scheduling Senhance cases for the most commonly performed surgeries without pressure from administration to constrain their robotic usage,” he said.The Senhance System, which has CE Mark, is being used commercially in Europe. Pope highlighted a recent system purchase by a large hospital in Germany and pointed to another site where a surgeon who first performed a case in early February is now using the system for four to five cases each week.
Despite these positives, the cost of capital equipment continues to be a hurdle for some hospitals. That has led TransEnterix to consider operating leases for some sites.
Pope explained, “In certain situations where we see high value and a long-term partnership with an institution, we have offered operating leases or other economic arrangements that will allow the hospital to immediately implement a Senhance Surgical Program.”
Sean Lavin, an analyst at BTIG, expressed concern about this strategy. “While we understand hospitals are under financial stress, it is concerning to us to see an early stage non-profitable company with limited cash begin to offer leases,” he wrote in a March 6 research note.
During the earnings call, Pope emphasized that operating leases wouldn’t be “a new normal.” Pointing out that the system’s selling prices have been comparable to the competitive offering, he added, “We just want to exhibit flexibility . . . we’re doing [an] operating lease or usually looking down for their next fiscal year for them to put aside the budget and then go ahead and pay on a typical ASP.”
Read More – Source: Surgical Robot Nearing FDA Submission | MDDI Medical Device and Diagnostic Industry News Products and Suppliers

