Medical device and MedTech insights, news, tips and more
TriGUARD 3 Cerebral Embolic Protection Device Receives CE Mark for Use in Transcatheter Heart Procedures
March 16, 2020
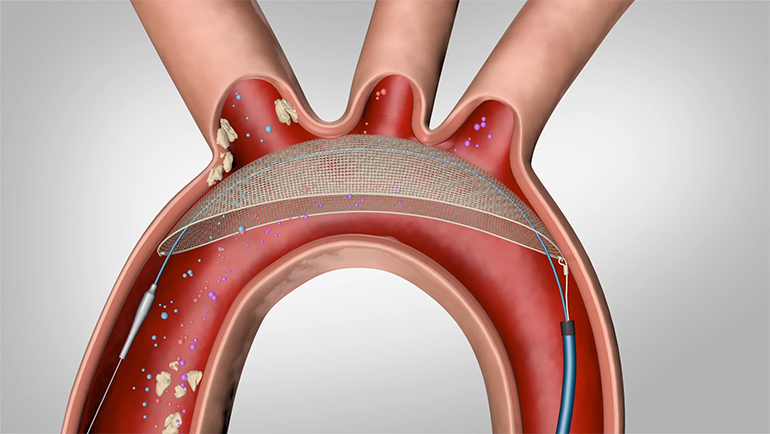
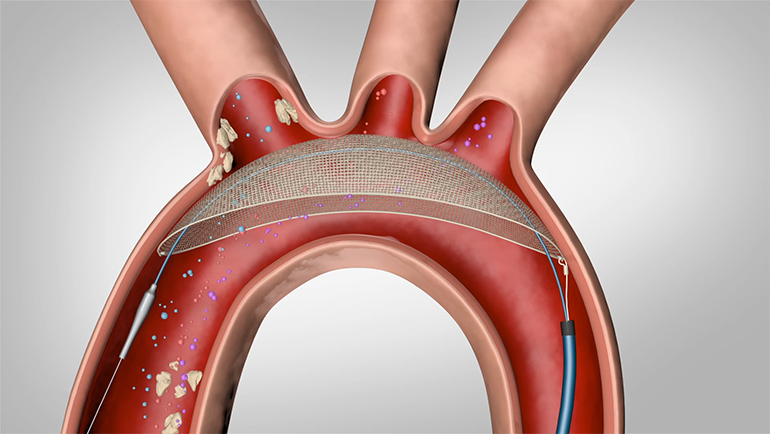
Keystone Heart Ltd., a Venus Medtech Company, today announced European CE Mark for the TriGUARD 3™ Cerebral Embolic Protection (CEP) Device. The device is designed to minimize the risk of cerebral damage by deflecting embolic debris away from cerebral circulation during Transcatheter Aortic Valve Implantation (TAVI) and other transcatheter heart procedures.
“Even with increased operator experience and availability of next-generation TAVI devices, cerebrovascular complications remain at a stable level, but continue to be the most feared and devastating complications during TAVI,” commented Doctor Nicolas Dumonteil, Interventional Cardiologist, Clinique Pasteur in Toulouse, France. “Interventional cardiologists are seeking options to avoid cerebral complications for their patients.”
The TriGUARD 3™ CEP device is the only CE Marked product designed to cover and protect all three major cerebral aortic arch vessels. The state-of-the-art Nitinol frame and dome-shaped mesh deflector are delivered transfemorally and designed to “self-position” in the aortic arch. This design allows the TriGUARD 3™ CEP device to conform to a variety of patient anatomies.
“Taking into consideration the devastating impact of stroke, we are pleased to bring this important technology to patients undergoing any transcatheter heart procedure. The introduction of the TriGUARD 3™ CEP Device in Europe provides physicians the only commercially available device that is designed to protect all three cerebral vessels.” Stated Chris Richardson, Keystone Heart, President and CEO.
Keystone Heart recently completed the REFLECT Trial (a pivotal randomized trial of the TriGUARD 3™ CEP device). Keystone Heart is currently finalizing data analysis ahead of the planned marketing application to the US Food and Drug Administration.
See Full Press Release: TriGUARD 3™ Cerebral Embolic Protection Device Receives CE Mark for Use in Transcatheter Heart Procedures
Written by: Keystone Heart Ltd.

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.