Medical device and MedTech insights, news, tips and more
Zero DBS Electrode Infections Reported When Utilizing the ClearPoint® NeuroNavigation System
April 20, 2016
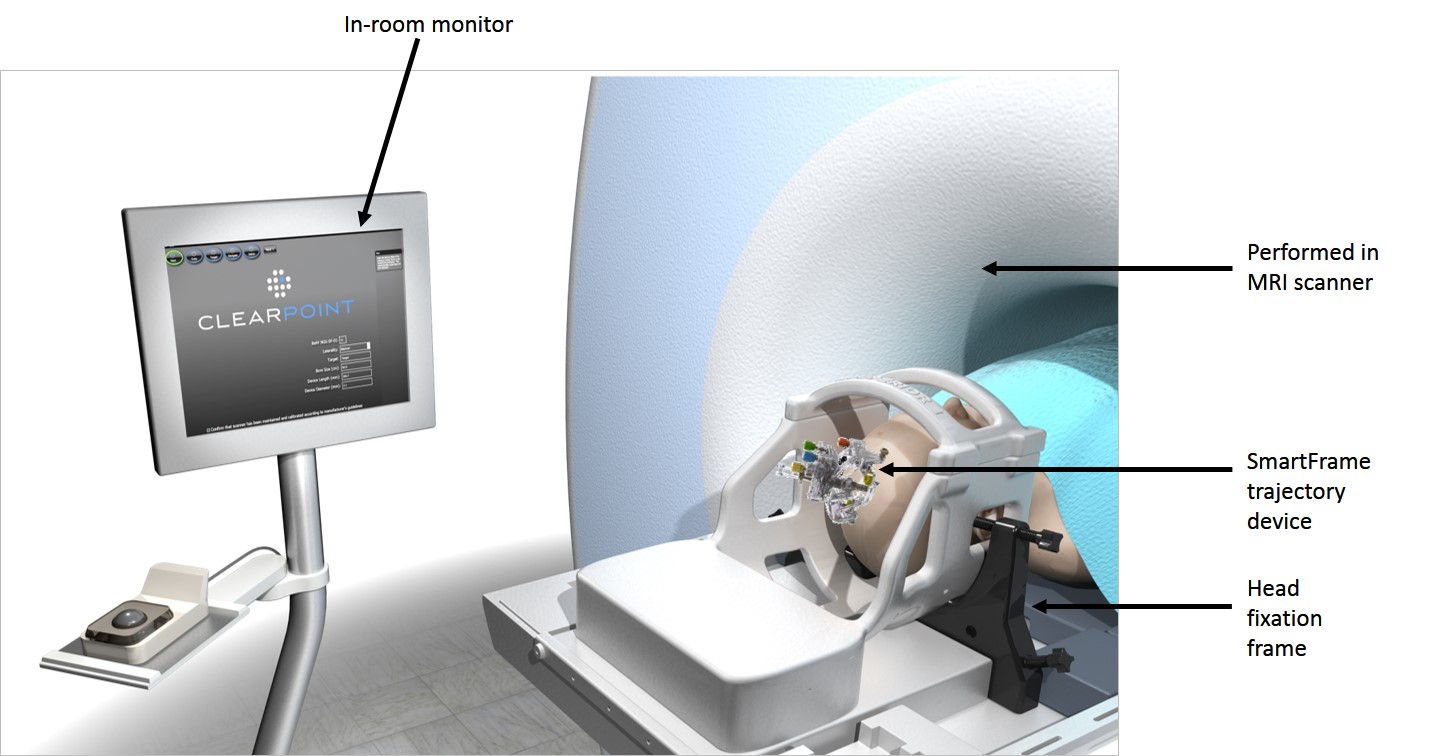
IRVINE, Calif., April 20, 2016 (GLOBE NEWSWIRE) — MRI Interventions, Inc. (OTCQB:MRIC) today announced the results of a 10 year study in which Deep Brain Stimulation (DBS) leads were implanted in a diagnostic MRI suite. The study was published in the March, 2016 issue of the Journal of Neurosurgery and outlines a 10 year history of 164 interventional MRI Deep Brain Stimulation procedures, in which 272 leads were implanted. All electrode placement procedures were performed at the University of California San Francisco (UCSF) Medical Center in a diagnostic 1.5T MRI scanner, and patients returned 1–3 weeks later to undergo placement of the implantable pulse generator (IPG) in a conventional operating room. After mid 2010 the electrode implant procedure was performed entirely in the diagnostic MRI suite using the ClearPoint NeuroNavigation System and accessories. There were zero infections related to electrode placement in all 115 electrode implant procedures using the ClearPoint System.
“The study’s finding of zero infections related to the electrode placement stage utilizing the ClearPoint NeuroNavigation is excellent,” stated Frank Grillo, Chief Executive Officer for MRI Interventions. “Also noteworthy is that the only infections reported in the study were either related to the implantation of the IPG or were associated with electrode placement or procedure preparation outside of the MRI suite, prior to ClearPoint’s commercial availability. We believe this paper is a great illustration of the safety of performing ClearPoint procedures in a diagnostic MRI suite.”
The ClearPoint system allows surgeons to plan, target, and adjust trajectories under real time MRI-guided visualization for placement of electrodes, catheters and biopsy needles. The entire procedure takes place in the MRI suite, and eliminates the need to move the patient to and from an operating room and an MRI suite.
“We were very pleased, although not surprised, to see these results indicating the absence of infections related to electrode placement in the MRI suite,” said Dr. Paul Larson, Professor and Vice Chair of Clinical Neurological Surgery at UCSF, and a co-author of the paper. “In addition, the overall clinical outcomes were comparable to those for conventional awake implantation approaches for electrode placement. Our experience with this procedure enables us to make the therapy available to a wider range of patients, including pediatric patients.”
An abstract of the paper can be read here: http://thejns.org/doi/abs/10.3171/2015.7.JNS15750
Source: Company News – MRI Interventions