Medical device and MedTech insights, news, tips and more
Zimmer Biomet Receives FDA Clearance for Robotic ROSA One Spine System
March 25, 2019
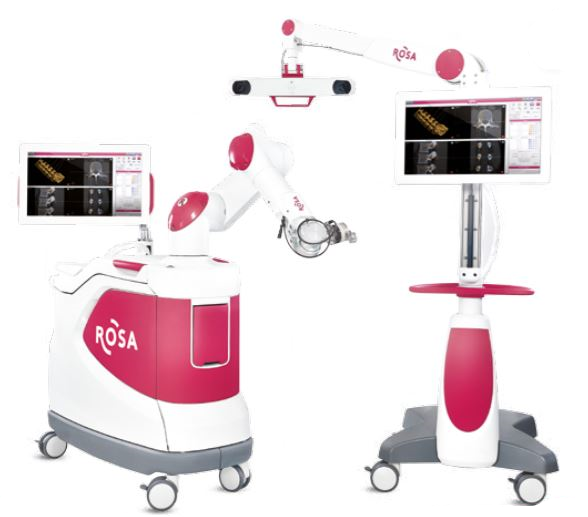
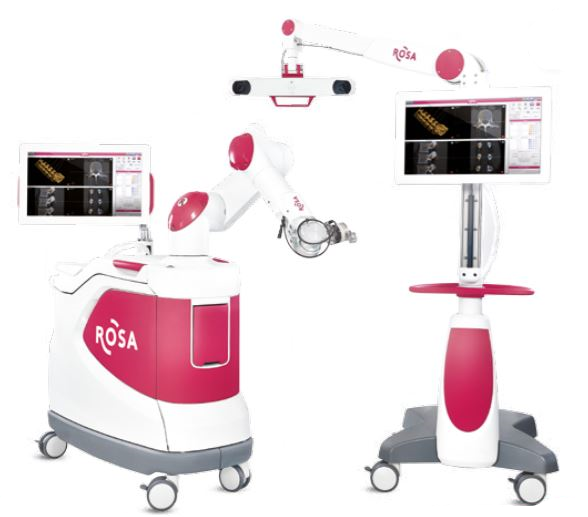
Zimmer Biomet Holdings, Inc., a global leader in musculoskeletal healthcare, today announced U.S. Food and Drug Administration 510(k) clearance of the ROSA® ONE Spine System for robotically assisted minimally invasive and complex spine surgeries, strengthening the Company’s comprehensive ROSA® ONE Brain and ROSA® Knee portfolio.
ROSA ONE Spine combines robotics and navigation while delivering a unique real-time patient ‘dynamic tracking’ capability. The platform features 3D intraoperative planning software in addition to a navigation suite of technologies designed to improve implant as well as instrument placement accuracy and predictability.
“ROSA ONE Spine functions as a dual robotics and navigation technology solution for minimally invasive and complex thoracolumbar spine procedures,” said Aure Bruneau, Zimmer Biomet’s Group President, Spine, CMF and Thoracic and Surgery Assisting Technology. “We are extremely excited about the addition of ROSA ONE Spine to our already released ROSA ONE Brain and ROSA Knee Systems.”
ROSA ONE Spine is a robotic and surgical navigation system designed to aid surgeons in performing thoracolumbar minimally invasive and complex spine procedures. ROSA ONE Spine is designed to accommodate each surgeon’s surgical flow. The ROSA ONE Spine System is now available on the same platform as ROSA ONE Brain and ROSA Knee, providing the only robot hardware platform on the market to treat neurosurgical, spinal and knee pathologies. The release of ROSA ONE Spine expands the applications of the ROSA ONE Brain System that received 510(k) clearance earlier this quarter and continues to leverage the robotics platform also utilized for Zimmer Biomet’s flagship orthopedics robot, the ROSA Knee System that received 510(k) clearance and CE Mark approval this quarter. ROSA ONE Spine features a combination of robotic guidance and navigation functionality at the surgeon’s request with a seamless and flexible workflow that ensures surgeons can focus on surgical goals and patient outcomes. The robot introduces ‘dynamic tracking’ functionality that allows the robot to move with the patient, providing accuracy without the need to be attached to the patient or the surgical table. The multi function robot can increase the utilization of the robotic platform within the neurosurgical and orthopedic department, decreasing technology acquisition costs and streamlining service, repair and education.
See Full Press Release at the Source: Zimmer Biomet Receives FDA Clearance of ROSA® ONE Spine System for Robotically-Assisted Surgeries
Press Release by Zimmer Biomet
 A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.