Medical device and MedTech insights, news, tips and more
ZOLL TherOx Receives CE Mark Approval for Supersaturated Oxygen Therapy
May 7, 2020
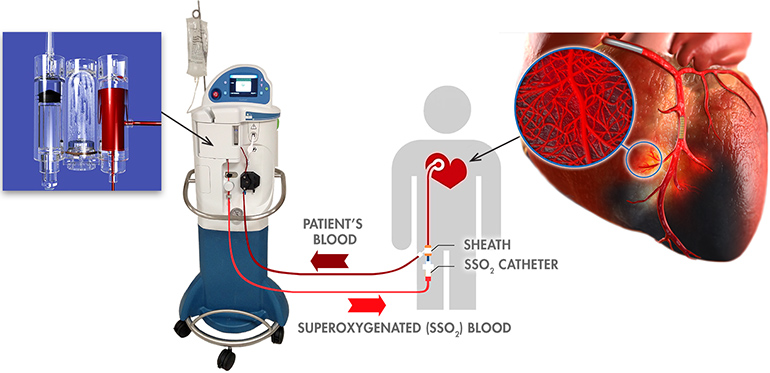
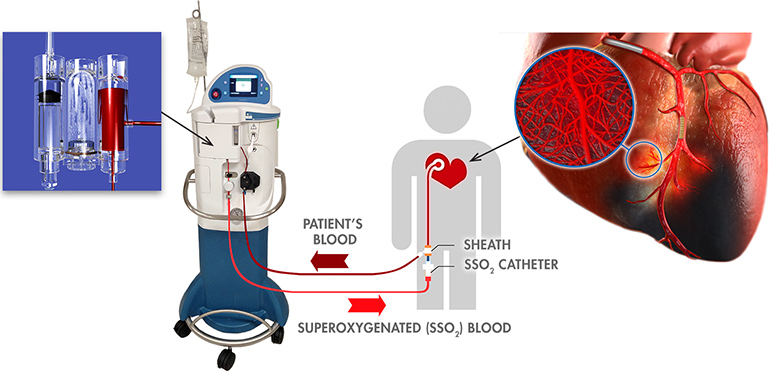
ZOLL Medical Corporation, an Asahi Kasei Group Company that manufactures medical devices and related software solutions, announced today it has received CE Mark approval to market and distribute its SuperSaturated Oxygen (SSO2) Therapy System in Europe. SSO2 Therapy provides interventional cardiologists with the first and only clinically proven treatment beyond percutaneous coronary intervention (PCI) to significantly reduce muscle damage in heart attack patients.
“SSO2 Therapy will support clinicians in delivering the highest quality of care for their STEMI patients in Europe and other countries that accept CE Mark,” said Neil Johnston, President, ZOLL Circulation.
SSO2 Therapy delivers hyperbaric levels of oxygen directly to the damaged heart muscle immediately after successful revascularization via angioplasty and stenting of the blocked coronary artery. It is indicated for patients who suffer the most serious kind of heart attack, left anterior descending ST-elevation myocardial infarction (LAD STEMI) — also known as a “widowmaker” due to the high mortality rate — and are treated within six hours of symptom onset. SSO2 Therapy is the first and only FDA-approved device beyond percutaneous coronary intervention (PCI) to reduce muscle damage in heart attack patients.
“I am very happy to know that ZOLL received CE Mark approval for the TherOx® System” said Antonio L. Bartorelli, MD, Interventional Cardiology Director at Centro Cardiologico Monzino, University of Milan. “We were among the first in Europe to observe the beneficial impact of SSO2 Therapy on left ventricular recovery after primary coronary intervention in patients with LAD ST-elevation myocardial infarction and we look forward to being able to use this effective treatment again in our patients.”
Historically, angioplasty and stenting have been the standard of care in treating heart attacks. Many patients do not achieve maximum clinical benefit and suffer from reduced heart function. More than 30% of severe heart attack patients develop heart failure, and of those, 50% will die within five years. SSO2 Therapy has been shown in prospective clinical trials to safely reduce infarct size in widowmaker heart attack patients. Decades of research on heart attack patients has demonstrated that infarct size reduction is correlated with reduced mortality and heart failure, and better left ventricular function.
See Full Press Release: ZOLL TherOx Receives CE Mark Approval for Supersaturated Oxygen Therapy
Written by: ZOLL

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.