Archive for May 2019
Medivis Wins FDA Clearance for Breakthrough Augmented Reality Surgical System
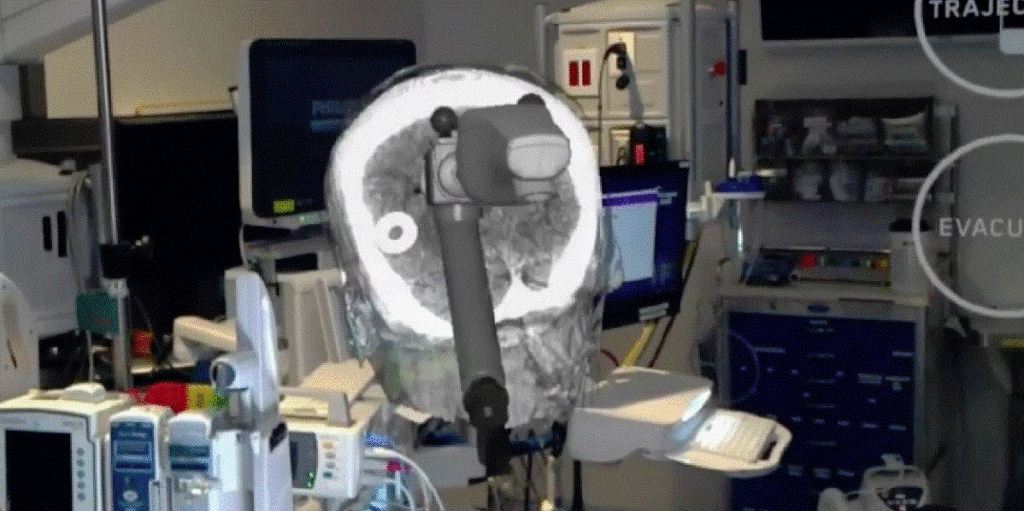
Medivis announced today that its groundbreaking augmented reality (AR) technology platform for surgical applications, SurgicalAR, has received 510(k) clearance for clinical use in the operating room by the U.S. Food and Drug Administration. The New York City based medical technology company will commence the immediate commercialization of the platform in the United States. The enterprise SurgicalAR platform integrates the…
Read MoreClearFlow Announces Positive Trial Results for PleuraFlow Thoracic Evacuation Device
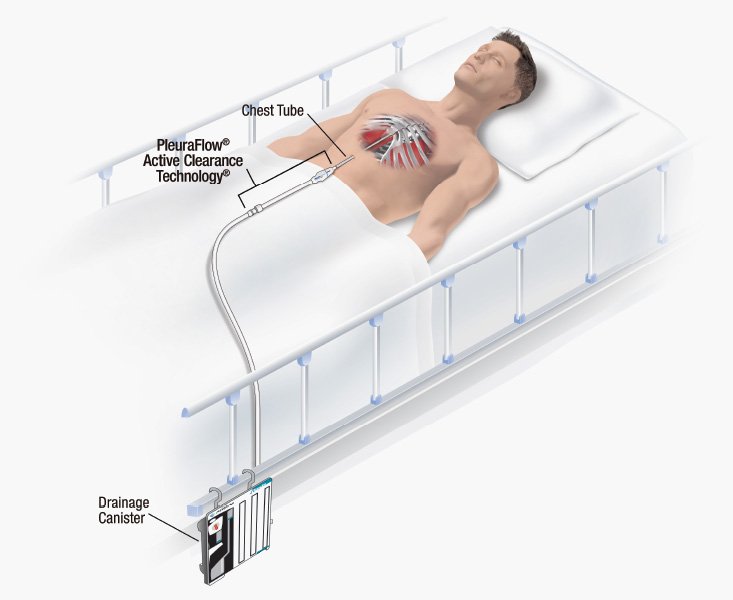
ClearFlow, Inc., a medical device company based in Anaheim, California, has announced that positive clinical trial results of its PleuraFlow® device were presented at the American Association for Thoracic Surgery (AATS) 99th Annual meeting on May 4 in Toronto, Canada. This new data stems from a trial evaluating the use of ClearFlow’s PleuraFlow® Active Clearance…
Read MoreMedtronic to acquire Titan Spine
Medtronic said last week that it agreed to acquire Titan Spine for an undisclosed amount. Mequon, Wis.-based Titan Spine makes a line of titanium interbody fusion devices featuring a surfacing technology it developed to encourage the ingrowth of bone into the implants. The acquisition is slated to close during Medtronic’s fiscal first quarter ending July 26,…
Read More