Archive for August 2019
First-ever: FDA Clears Biobeat’s Wearable Watch and Patch for Non-invasive Cuffless Blood Pressure Monitoring
Biobeat, a bio-medical technology company developing advanced sensing and remote monitoring solutions for patients, announced today that the U.S. Food and Drug Administration (FDA) has granted a 510K clearance for its patch and watch for measurement of blood pressure, oxygenation and heart rate in hospitals, clinics, long-term care and at home. Biobeat’s products enable cloud-based healthcare…
Read MoreMedtronic’s Ishrak Set to Retire, Leadership Succession Announced
Medtronic announced that Omar Ishrak, Medtronic’s Chairman and Chief Executive Officer (CEO), has announced his intention to retire as CEO on April 26, 2020, following the end of the company’s current fiscal year. As a result, the Medtronic Board of Directors announced key leadership appointments as part of its multi-year, leadership succession planning process, which…
Read MoreFDA Approves Alcon’s AcrySof IQ PanOptix trifocal IOL
Alcon won FDA approval for its AcrySof IQ PanOptix trifocal intraocular lens and is preparing an initial commercial launch for the device. The Fort Worth, Texas-based company’s AcrySof IQ PanOptix device is the first and only trifocal device in the U.S. for patients undergoing cataract surgery. It’s designed to provide a combination of improved near,…
Read MoreOneDraw A1C Needle-Free Test System FDA Cleared
Drawbridge Health, out of Menlo Park, California, won FDA clearance for its OneDraw A1C Test System, which consists of the OneDraw Blood Collection Device and the OneDraw A1C Test. Designed for use by clinicians, the disposable product is used to draw, collect, and stabilize blood to measure hemoglobin A1c (HbA1c) levels to help manage glucose…
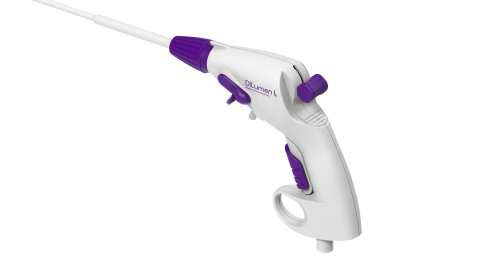
Read MoreLumendi Gets FDA Clearance for Endolumenal Interventional Knife
Westport, Connecticut-based medical device innovator Lumendi, LLC (http://www.lumendi.com) has announced it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for the DiLumen Ik™ Endolumenal Interventional Knife, a sterile, single-use, disposable monopolar electrosurgical device for cutting, dissecting, and cauterizing tissue within the digestive tract during endoscopic procedures. The DiLumen Ik™ is also designed to deliver a…
Read MoreMiracor’s Heart Attack Device Secures FDA Breakthrough Status
The concept of pressure-controlled intermittent coronary sinus occlusion, abbreviated to PICSO, has been around since the 1980s. PICSO entails placing a device in the coronary sinus to intermittently obstruct blood flow. Through the interference, PICSO may increase blood flow to parts of the heart affected by the myocardial infarction. Miracor, which received a CE mark for its…
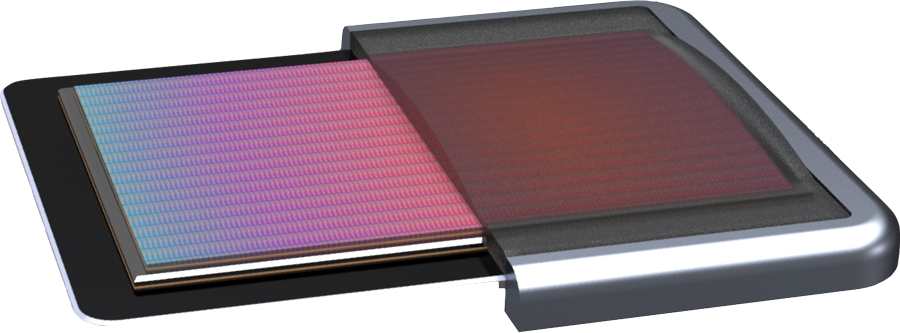
Read MoreExo Imaging Emerges from Stealth to Announce All-In-One Handheld Ultrasound Platform, Funding
Exo Imaging, Inc., a company pioneering a high-performance ultrasound platform based on patented Piezoelectric Micromachined Ultrasonic Transducers (pMUT) technology and artificial intelligence (AI) for imaging and therapeutic applications, announced a $35M Series B financing round. The company was founded in 2015 and has raised nearly $50M to date. Intel Capital led the Series B. Other investors…
Read MoreFDA Approves CVRx’s Neuromodulation Device for Heart Failure
CVRx, Inc., a private medical device company, announced today that it has received Premarket Approval (PMA) from the United States Food and Drug Administration (FDA) to market its BAROSTIM NEO device for heart failure in the United States. The FDA’s Center for Devices and Radiological Health (CDRH) approved the Company’s submission after a thorough review of…
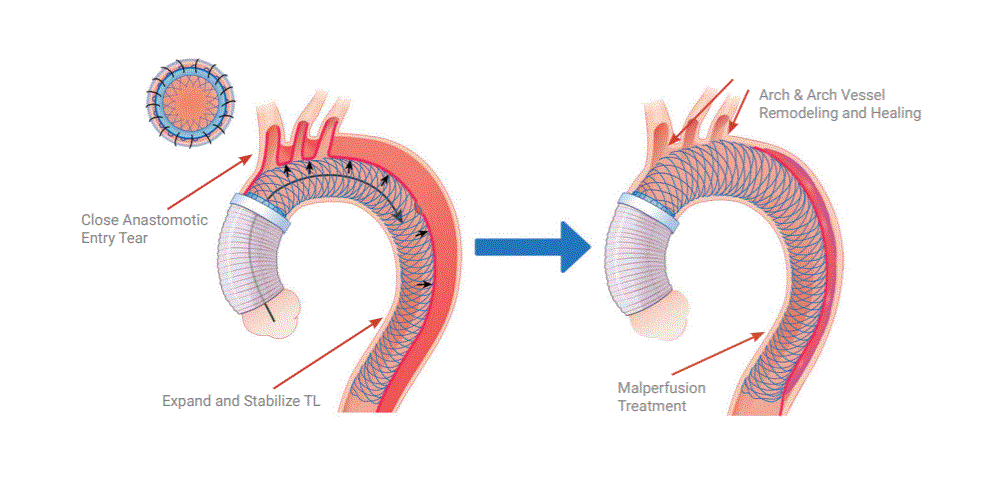
Read MoreFDA Grants Breakthrough Device Designation to Ascyrus Medical Dissection Stent
Ascyrus Medical announced yesterday that it has received Breakthrough Device Designation from the FDA for its Ascyrus Medical Dissection Stent (AMDS) to treat acute Type A aortic dissections. This designation serves as validation of the clinical importance of the AMDS and represents a significant milestone in the continued advancement of the technology globally. Ascyrus Medical…
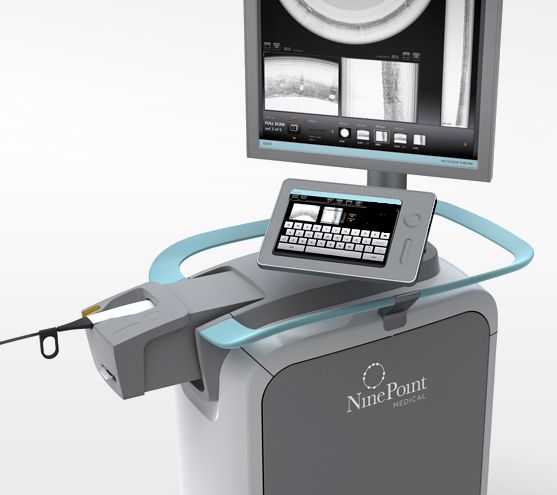
Read MoreFDA Clears Ninepoint’s OCT Imaging System for Use in Bile Ducts
NinePoint Medical, Inc., a transformative medical device company pioneering the use of a real-time optical imaging platform for gastrointestinal applications, announced today that it has received U.S. Food and Drug Administration (FDA) clearance to market the NvisionVLE® Imaging System for use in the pancreas and bile duct. These anatomical indications add to the previously existing esophageal…
Read More