Archive for February 2021
NuVasive Acquires Simplify Medical
NuVasive, Inc., the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally integrated solutions, today announced that it has acquired Simplify Medical, a privately held company and developer of the Simplify® Cervical Artificial Disc (Simplify Disc) for cervical total disc replacement (cTDR). The acquisition of Simplify Medical adds the most clinically…
Read MoreOpSens Announces Closing of $28.75 Million Bought Deal Public Offering Including $3.75 Million Over-Allotment Option Exercised in Full
OpSens Inc. (“OpSens” or the “Company”) (TSX:OPS) (OTCQX:OPSSF) announced last week the closing of its previously announced bought deal public offering (the “Offering”) of common shares of the Company (the “Common Shares”), for total gross proceeds of approximately $28,750,000. The Company issued an aggregate of 15,972,222 Common Shares, at a price of $1.80 per Common Share,…
Read MoreFusion Robotics Wins FDA Clearance for 3D Imaging Robotic Targeting System
Fusion Robotics LLC, a spinal robotics and navigation company today announced receiving 510(k) clearance to market their 3D imaging compatible navigation and robotic targeting system for spine surgery in the U.S. market. The Fusion Robotics System addresses the key limitations of current spinal navigation and robotics systems by offering greater procedural efficiency with significantly less…
Read MoreFDA clears Signifier Medical’s Electric Tongue Muscle Strengthener to Treat Sleep Apnea and Snoring
The FDA has approved eXciteOSA, the revolutionary first-ever daytime treatment for mild obstructive sleep apnea and snoring. Used for only 20 minutes per day for a period of six weeks and then twice per week, the therapy is clinically proven to improve the quality of sleep by significantly reducing obstructive sleep apnea and snoring. Signifier…
Read MoreImplicity Rolls Out AI-Empowered Remote Cardiac Monitoring to Over 10,000 Patients
Implicity, the leading remote cardiac monitoring company in Europe, officially announced its rollout into the US market via its partnership with the Phoenix-based company, IronRod Health. Implicity and IronRod have been nominated for The Prix Galien Medstartup USA in the category of “Best collaboration in the Medtech and Digital health sector”, organized by the Galien…
Read MoreVascular Perfusion Solutions Lands FDA Breakthrough Designation for Organ-Transport Device
The U.S. Food and Drug Administration (FDA) has granted Vascular Perfusion Solutions, Inc. (VPS) a Breakthrough Device Designation for its groundbreaking VP.S Encore™ oxygenated perfusion cardiac transport device. The patent-pending technology uses oxygen to enable preserving vascularized tissue for eight hours, and more, doubling the viability of organs beyond the current standard of care. As…
Read MorePuzzle Medical Receives FDA Breakthrough Device Designation for Transcatheter Heart Pump
Puzzle Medical Devices Inc., (www.puzzlemed.com) announced today that the U.S. Food and Drug Administration (FDA) has granted the company a Breakthrough Device Designation for its revolutionary transcatheter pump to address heart failure. The FDA Breakthrough Device Program is intended to help patients receive more timely access to certain medical devices that have the potential to…
Read MoreAcuitive Technologies Wins FDAClearance for Tendon Interference Screw System
Acuitive Technologies announced last week that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market the CITREFIX™ Knotless Suture Anchor System with CITREGEN™ material technology, a new generation bioresorbable synthetic polymer. The CITREFIX system is intended to assist the attachment of tissue to bone during orthopedic surgeries such as…
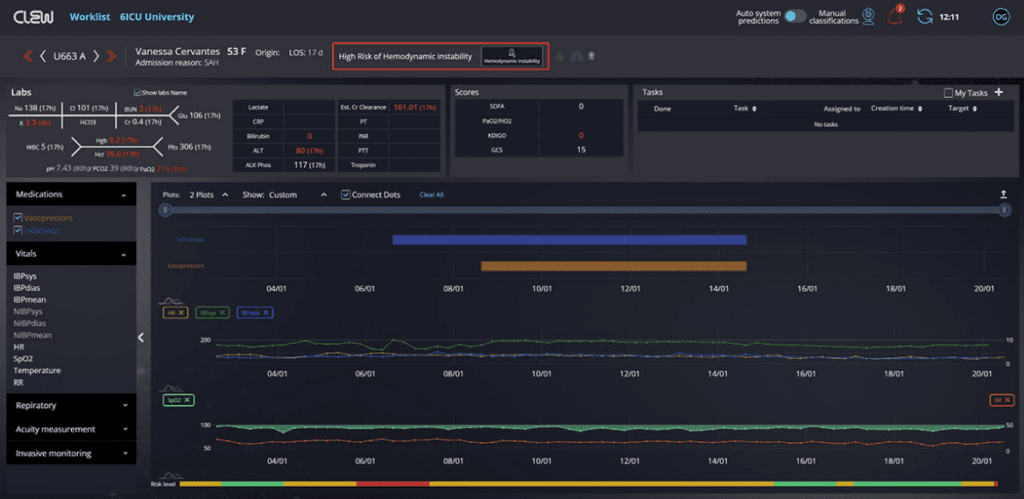
Read MoreCLEW Medical Receives FDA Clearance for AI-Based Software that Predicts Hemodynamic Instability
CLEW Medical, a leader in AI-powered predictive analytics, today announced that the U.S. Food and Drug Administration (FDA) has given 510(k) clearance and authorized the use of “CLEWICU,” CLEW’s artificial intelligence (AI) based ICU solution, to predict hemodynamic instability in adult patients. The clearance is the FDA’s first for such a device, and follows the…
Read MoreInovytec’s Small “Sparrow” Ventilators Receive FDA Clearance
Inovytec, an innovator of multi-functional and user-friendly critical medical devices, announced today that it was granted FDA 510(k) clearance to market and sell its Ventway Sparrow ventilators in the United States. The ventilators are already commercialized in Europe, Canada and Australia and undergoing registration procedures in other countries. The Ventway Sparrow transport and emergency ventilators are designed for mobility, high-performance…
Read More