Archive for November 2020
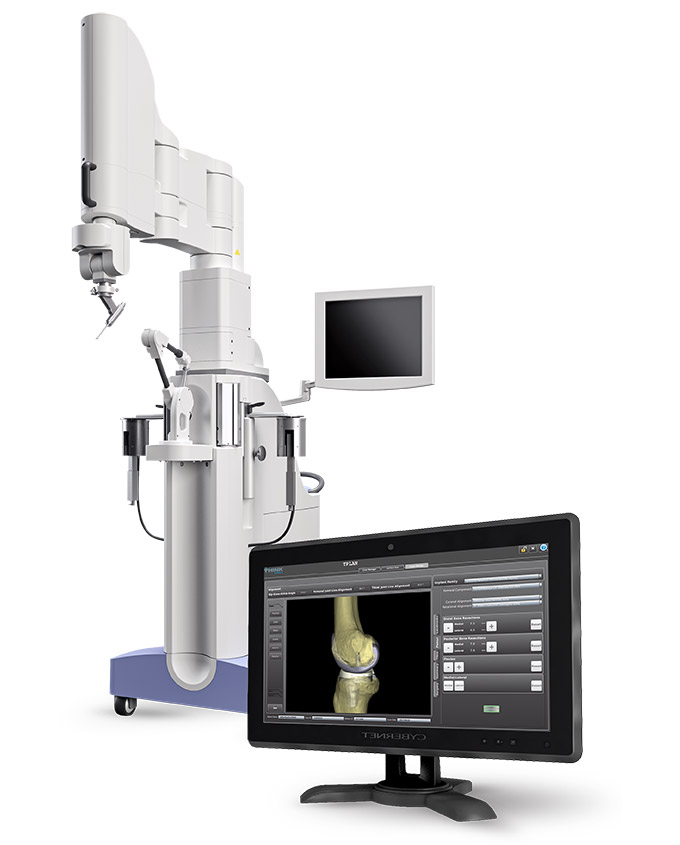
THINK Surgical Receives FDA Clearance of Second-Generation Active Robot
THINK Surgical, an innovator in the field of orthopedic active robot surgery, announced today that the Food and Drug Administration (FDA) has cleared the second-generation of the TSolution One Total Knee Application. The system features an active robot for total knee replacement, providing fully automated bone preparation, and gives surgeons a choice of implant options.…
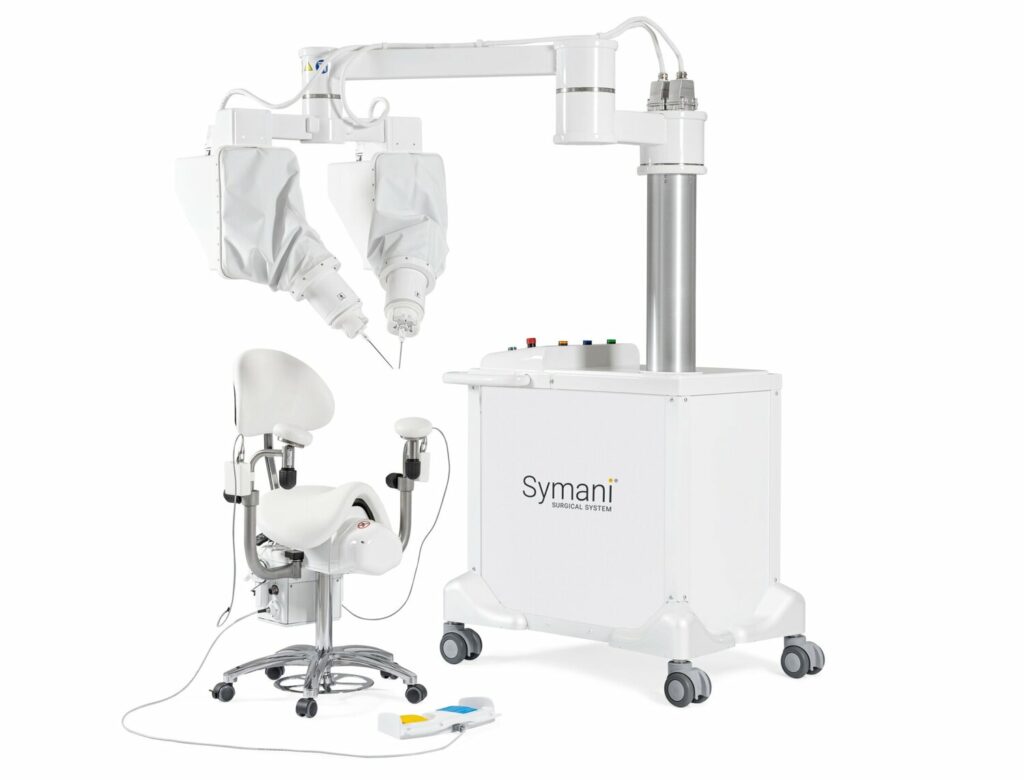
Read MoreMMI’s Symani Robotic Microsurgery System Cleared in Europe
MMI SpA, an Italian company dedicated to improving clinical outcomes for patients undergoing microsurgery, announced today the CE Mark, launch and first human use of its Symani® Surgical System in Europe for open microsurgical procedures. The first four robotic surgeries were successfully performed in Florence, Italy, including three complex, post-traumatic lower limb reconstructions as well…
Read MoreAllotrope Medical Announces FDA Clearance of StimSite
Allotrope Medical™ Inc., a company committed to advancing surgical safety and precision, today announced its FDA clearance of their device, StimSite. StimSite provides ob/gyns, general and colorectal surgeons the new ability to use their existing surgical instruments to help locate and identify ureters using electrical stimulation. Ureter identification is a critical step in safely advancing…
Read MoreAdagio Medical Raises $42.5 Million In Series E Financing
Adagio Medical, Inc., a leading innovator for the treatment of atrial fibrillation (AF) and ventricular tachycardia (VT), and developer of the intelligent Continuous Lesion Ablation System (iCLAS™), today announced that it has closed a $42.5 million Series E equity financing. Proceeds from the financing will be used to support the ongoing iCLAS™ Investigational Device Exemption (IDE) trial, accelerate the European VT CE-Mark…
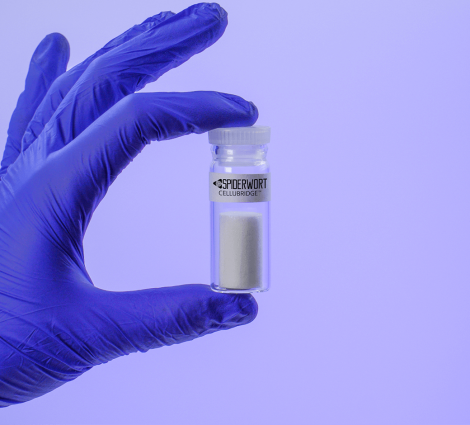
Read MoreFDA Grants Breakthrough Device Designation for Spiderwort Spinal Cord Technology
Spiderwort Inc., a Canadian medical device company developing innovative biomaterials for regenerative medicine, is pleased to announce that the U.S. Food and Drug Administration (FDA) has designated CelluBridgeTM, Spiderwort’s Spinal Cord Scaffold Implant, as a “Breakthrough Device”. The FDA Breakthrough Devices program creates a path for innovators to get their medical devices to market faster. The program…
Read MoreFreudenberg Medical Opens New Global Headquarters and Facility Expansion in Massachusetts
Freudenberg Medical, member of the Freudenberg Group and a global developer and manufacturer of specialty components and finished devices for the medical device industry, has completed construction of a new medical manufacturing operation in Beverly, Massachusetts. Freudenberg Medical has established the facility as its new global headquarters and the location will manufacture medical devices and components in…
Read MoreItamar Medical Wins National Sleep Foundation 2020 SleepTech Award
The National Sleep Foundation (NSF) named Itamar Medical Ltd. (NASDAQ and TASE: ITMR) winner of the 2020 SleepTech Award. The SleepTech Award recognizes the year’s most innovative efforts in advancing sleep technology. Itamar Medical is a technology company focused on the development and commercialization of non-invasive medical devices and solutions to aid in the diagnosis of…
Read MoreCerapedics Announces Commercial Launch of i-FACTOR+ Matrix in Canada
Cerapedics, a private ortho-biologics company, today announced the full commercial availability of i-FACTOR+ MATRIX for surgical implantation in Canada. “We are excited to announce that i-FACTOR+ Matrix is now fully available in Canada through our distributor Surgi-One and can be used in both spine and general orthopedic applications,” said Glen Kashuba, Chief Executive Officer of Cerapedics. “Canada will be the…
Read MoreStryker Will Divest Total Ankle Business to get FTC Blessing for Wright Medical Acquisition
The Federal Trade Commission will require medical device companies Stryker Corp. and Wright Medical Group N.V. to divest all assets related to Stryker’s total ankle replacements and finger joint implant products to remedy concerns that Stryker’s proposed $4 billion acquisition of Wright will harm competition in those markets. Under the terms of the consent agreement, the companies…
Read More