Archive for March 2021
Rapid Medical Receives FDA Clearance for the First Adjustable Stent Retriever for Use in Ischemic Stroke Treatment
Rapid Medical, developer of responsive, adjustable neurovascular devices, announces the FDA clearance of its TIGERTRIEVER™ revascularization device for use in the treatment of ischemic stroke. TIGERTRIEVER is the first stent retriever to offer intelligent control, enabling neuro interventionalists to better remove blood clots and restore blood flow the brain following an ischemic event—a condition that devastates 800,000…
Read MoreSeaSpine Acquiring 7D Surgical for $110M
SeaSpine Holdings Corporation (NASDAQ: SPNE), a global medical technology company focused on surgical solutions for the treatment of spinal disorders, announced today that it has entered into an agreement to acquire all of the issued and outstanding shares of 7D Surgical, Inc., a privately-held, Toronto-based company, in a cash and stock deal valued at $110 million, subject to customary…
Read MoreCatalyst OrthoScience Receives FDA 510(k) Clearance of Its Reverse Shoulder System
Catalyst OrthoScience Inc. (Catalyst), a medical device company focused on the upper extremity orthopedics market, has received clearance from the U.S. Food & Drug Administration (FDA) to market its reverse shoulder system. Catalyst expects to begin a limited user release in the United States in the second quarter of 2021 followed by a commercial launch later…
Read MoreQardio Lands 510(k) for Ambulatory ECG Device
Qardio, Inc. announced today that the U.S. Food and Drug Administration (FDA) has granted the company 510k clearance for its QardioCore ambulatory ECG device. QardioCore will initially be marketed for holter monitoring applications, for use with QardioMD, Qardio’s cloud-based remote patient monitoring solution. QardioCore extends Qardio’s remote patient monitoring platform from primary care and hypertension monitoring to acute…
Read MoreImbio Receives FDA 510(k) Clearance for New Cardiothoracic Imaging Algorithm
Imbio, a leading provider of artificial intelligence (AI) solutions for medical imaging analysis, has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its RV/LV Analysis™ algorithm. The RV/LV Analysis algorithm is a rapid, automated assessment of potential right ventricular dilation. The tool quickly and accurately measures the ventricles of the heart to provide the ratio of…
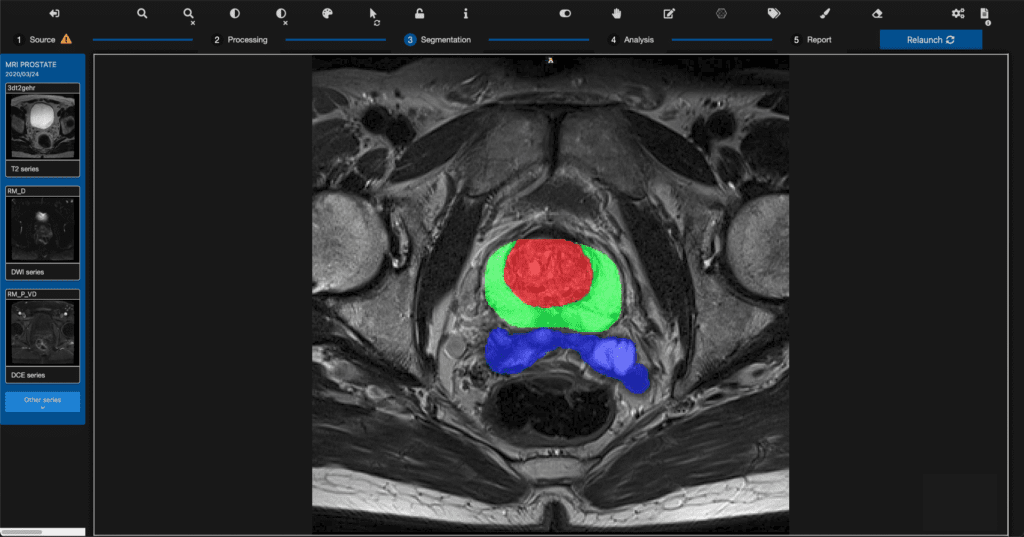
Read MoreQuibim Receives FDA 510(k) Clearance for qp-Prostate AI Solution for Prostate MRI Analysis
Quibim, a global leader in whole-body medical imaging analysis, announced today the launch of qp-Prostate, its latest and most advanced prostate AI based Magnetic Resonance (MR) solution, after receiving 510(k) clearance by the US Food and Drug Administration. The solution aids in the process of prostate magnetic resonance imaging (MRI) reporting from visualization to quantification with…
Read MorePreceptis Medical Announces Publication of Positive Results from Pediatric In-Office Study of the Hummingbird Tympanostomy Tube System (TTS)
Preceptis Medical, Inc., a company dedicated to helping ENT surgeons improve how they care for children, today announced positive results from a prospective, multicenter study designed to assess in-office pediatric ear tube placement with the FDA-cleared Hummingbird Tympanostomy Tube System (TTS). Peer-reviewed results from the study were published in Laryngoscope Investigative Otolaryngology. Outcomes from the study…
Read MoreAccess Vascular Inc. Secures $20 Million in Series B Financing
Access Vascular, Inc., a company addressing the most common and costly venous access complications, today announced it has closed on a Series B round of financing. TVM Capital Life Science led the round with a $15 million commitment, with existing investors also participating. Access Vascular has developed a proprietary biomaterial platform that is both hydrophilic and lubricious,…
Read MoreShockwave Intravascular Lithotripsy FDA Approved to Treat Advanced Heart Disease
Shockwave Medical, Inc., a pioneer in the development of Intravascular Lithotripsy (IVL) to treat severely calcified cardiovascular disease, announced last month that the company’s sonic pressure wave therapy received Pre-Market Approval for severely calcified coronary artery disease from the U.S. Food and Drug Administration (FDA). The innovative technology, which was granted Breakthrough Device designation by…
Read MoreFDA Grants Breakthrough Device Designation for Anuncia’s Cerebral Spinal Fluid Treatment
Anuncia Inc., an emerging leader in Cerebral Spinal Fluid (CSF) management, received U.S. Food and Drug Administration (FDA) Breakthrough Device Designation for its ReFlow™ System Mini intended for the treatment of CSF disorders requiring shunting such as hydrocephalus, a debilitating and life-threatening condition affecting more than 1 million U.S. patients. Elsa Abruzzo, President of Anuncia Inc., stated,…
Read More