Archive for April 2023
Kerecis Combines Fish-Skin Graft and Silicone Cover for Wound Treatment
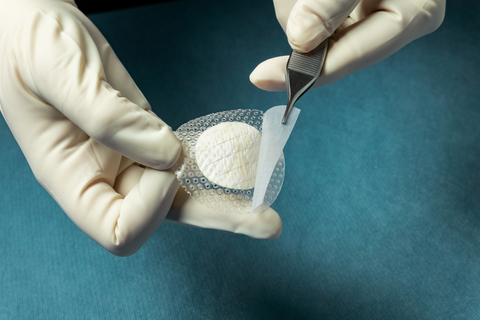
Kerecis® today announced MariGen® Shield, which integrates the company’s proven fish-skin graft with a silicone contact layer for treating chronic and complex wounds. The medical-fish-skin company also announced the results of a clinical study comparing the effectiveness of the Kerecis fish-skin grafts to a standard of care for diabetic foot ulcers. Both announcements were made at the Symposium…
Read MoreInnoblative Receives U.S. FDA Breakthrough Device Designation for its SIRA RFA Electrosurgical Device
Innoblative Designs, Inc. (Innoblative), a private medical device company addressing clinical unmet needs for patients with breast cancer, announced that it received Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA) for the company’s SIRA™ RFA Electrosurgical Device (SIRA). The SIRA device is intended for use in breast cancer patients undergoing BCS, commonly…
Read MoreCardieX Receives FDA 510(K) Clearance for a World-First Vascular Biometric Monitor – the CONNEQT Pulse
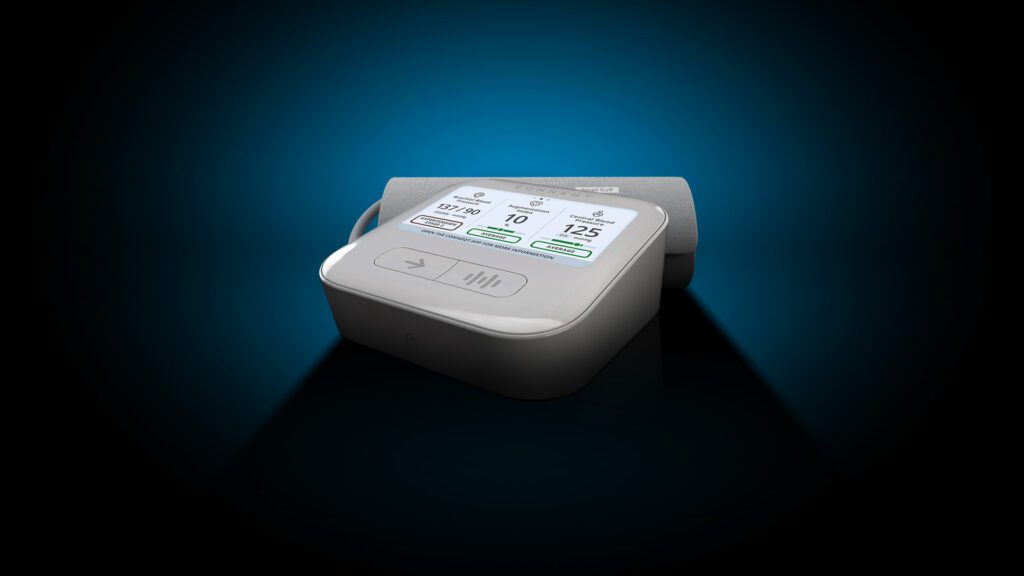
CardieX Limited (ASX: CDX) (CardieX, the Company), today announced its new arterial health monitor, the CONNEQT Pulse (Pulse), has received 510(k) clearance from the U.S. Food and Drug Administration (FDA). Pulse is the only vital signs monitor targeted at home, clinician, and clinical trial use that provides measurements of both brachial blood pressure (the pressure at…
Read MoreFirst Light Field-Enabled Spine Surgery Navigation Platform, Proprio Paradigm™, Receives FDA Clearance
Proprio, a Seattle-based medical technology company, today announced its surgical navigation platform, Paradigm, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA). Paradigm utilizes an advanced approach to replace traditional surgical navigation technologies that pull attention away from the patient and disrupt workflows in the process. Proprio’s Paradigm platform is the first to…
Read MoreBlackSwan Vascular Wins FDA Premarket Approval of Innovative New Treatment for Peripheral Arterial Hemorrhages
Bay Area-based BlackSwan Vascular, Inc., a privately held company that is developing innovative therapies in endovascular embolization, is pleased to announce it has received FDA Premarket Approval (PMA) of its Lava® Liquid Embolic System (Lava® LES) for treatment of peripheral arterial hemorrhage. Lava LES, a nonadhesive injectable, is the first liquid embolic product approved by…
Read MoreRapidAI Receives First and Only FDA 510(k) Clearance of Non-Contrast CT Imaging Product to Accelerate Acute Stroke Triage
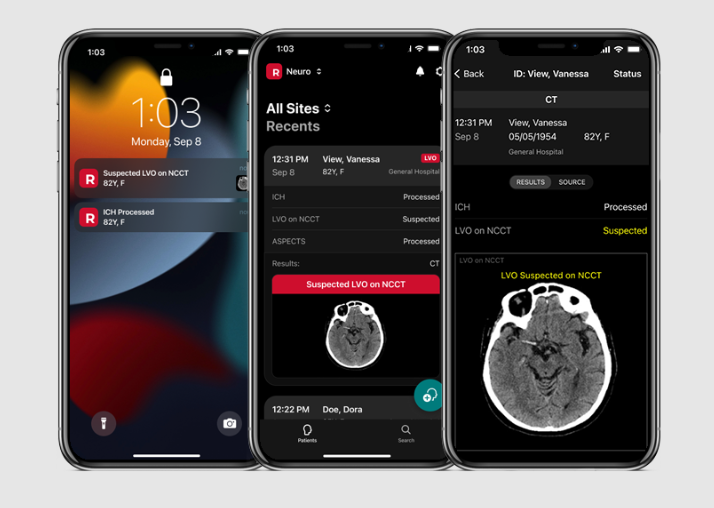
RapidAI, the global leader in neurovascular and vascular AI-enhanced clinical decision support and patient workflow, today announced it has received FDA 510(k) clearance for Rapid NCCT Stroke – a major addition to the RapidAI suite of non-contrast based solutions for stroke and trauma care, and the first and only FDA cleared medical device to detect suspected intracranial…
Read MoreCaristo Diagnostics Completes Series A Financing and Welcomes New CEO to Advance Ground-Breaking AI Technology for Cardiac Disease Detection
Caristo Diagnostics Limited, a global leader in cardiac and vascular disease diagnostics and risk prediction, today announced it has secured a further £13 million (US$16.3 million) to conclude its Series A financing round. Caristo will use the capital to advance its CaRi-Heart® technology, an AI-assisted diagnostics and risk prediction tool, into standards of cardiac care in…
Read MorePrecision Optics Appoints Mahesh Lawande as Chief Operating Officer
Precision Optics Corporation, Inc. (NASDAQ: POCI), a leading designer and manufacturer of advanced optical instruments for the medical and defense industries, today announced the appointment of medical device and aerospace/defense industry manufacturing veteran, Mahesh Lawande, as the Company’s Chief Operating Officer effective April 24, 2023. In the newly created role, Lawande will lead Precision Optics operations team, including…
Read MoreNeuvotion Secures an Additional $1.25M to Commercialize NeuStim™ Technology for Treating Stroke and Debilitating Injuries
Neuvotion, Inc. is an early-stage medical device company developing neurostimulation products for the rehabilitation, brain-computer interface, and physical therapy markets, today announced that it has received an additional $1.25M in funding, bringing cumulative funding to over $2.75M. Northwell Holdings, the venture investment arm of Northwell Health, Topspin Fund, and Long Island Angel Network also participated in the round. This…
Read MoreSentiAR Announces Close of $8.5 million Series B Financing
SentiAR, Inc. a St. Louis, Missouri based pioneer in using Augmented Reality (AR) visualization technology for medical procedures, has closed an $8.5 million Series B financing. The financing was led by cultivate (MD) Ventures and joined by MedVenture Partners alongside several insider investors, including TechWald Holding, VCapital, QRM Capital, and Harmonix Fund. The funding will allow the company to launch…
Read More