Orthopedics and Spine
FDA clears Intelivation Technologies new Hammerdesis™ Interphalangeal Fusion System
Intelivation Technologies, an innovative medical device company with a cutting-edge orthopedic portfolio announced today that it has received clearance from the Food and Drug Administration for its Hammerdesis™ Interphalangeal Fusion System. Hammerdesis™ allows surgeons to correct hammertoe deformities and degenerative issues in patients by simply affixing a distinctively designed implant on the patient’s toe joint. This one-of-a-kind…
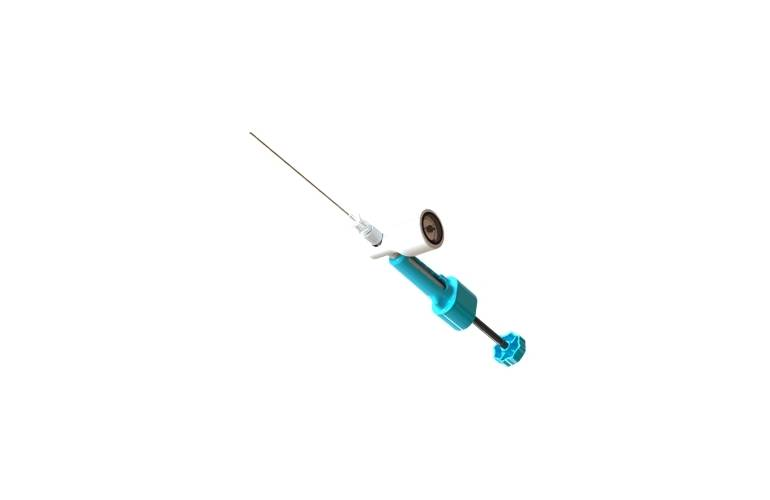
Read MoreThe FDA Approves IDE for ReGelTec’s Pivotal Study of HYDRAFIL® for Chronic Low Back Pain due to Degenerative Disc Disease
ReGelTec, Inc., announced that the U.S. Food and Drug Administration has approved an IDE for the company’s pivotal study to support premarket approval of its HYDRAFIL® System. The HYDRAFIL System contains an injectable polymer that is implanted percutaneously via a needle to augment the native disc in a procedure performed under local anesthesia at an…
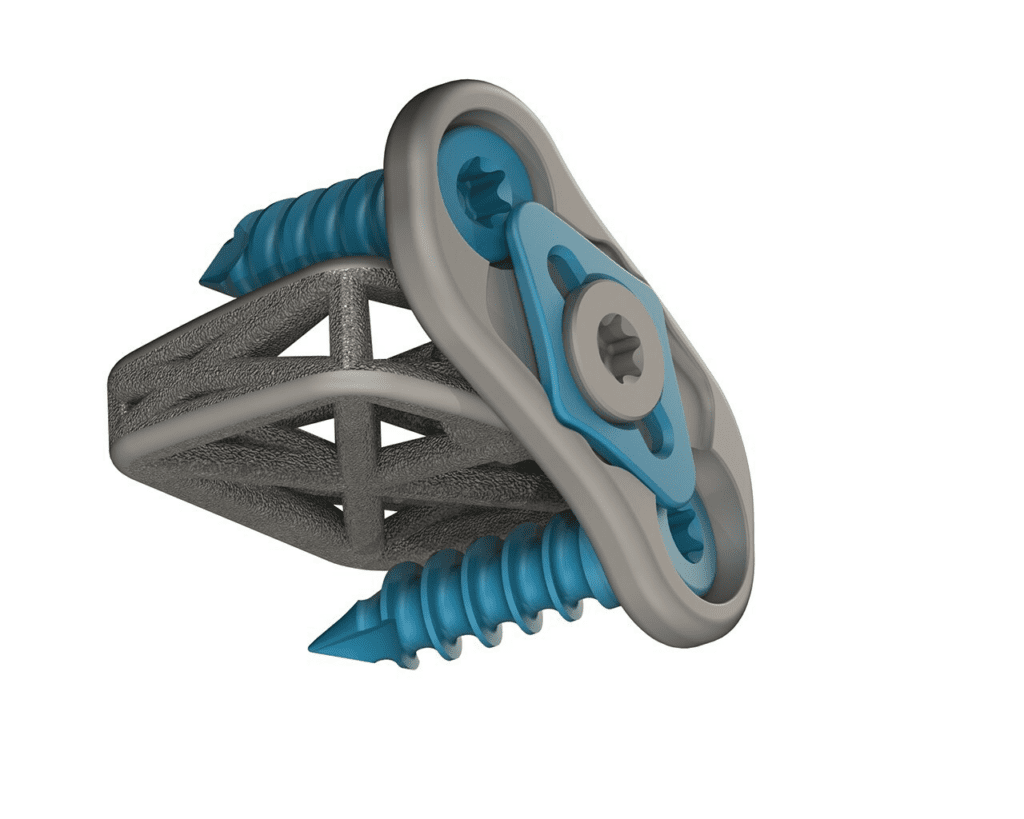
Read More4WEB Medical Receives 510k Clearance to Market New Integrated Cervical Plate
4WEB Medical, an orthopedic implant company focused on developing innovative implants that utilize its proprietary Truss Implant Technology™, announced that it has received 510K clearance to market the newest additions to the company’s implant portfolio, the Cervical Spine Truss System (CSTS) Integrated Plating Solution and two cervical interbody line extensions which include implants with 12 degrees of…
Read MoreChoiceSpine® Announces Standalone Indication for Blackhawk® Ti 3D Printed Cervical Spacer System
ChoiceSpine LLC, a privately held spinal device company held by Altus Capital Partners in Knoxville, TN, is pleased to announce today that it has received clearance from the U.S. Food and Drug Administration (FDA) to market the Blackhawk® Ti 3D Printed Cervical Spacer System with standalone clearance. Blackhawk® Ti utilizes preassembled integrated anchor technology with a proven…
Read MoreOrthofix Announces Leadership Changes
Orthofix Medical Inc. (NASDAQ: OFIX), a leading global spine and orthopedics company, today announced that Catherine Burzik, Chair of the Orthofix Board of Directors, has been appointed Interim Chief Executive Officer; Geoffrey Gillespie, Orthofix Vice President, Corporate Controller, has been appointed Interim Chief Financial Officer; and Puja Leekha, Orthofix Senior Vice President, Chief Ethics and…
Read MoreBionicM Earns FDA Registration and Class II Exempt Device Listing for Bio Leg™; Its New Motor-Robotic Prosthetic Knee
BionicM, an innovative Japanese medical device startup, is pleased to announce that it has received U.S. Food and Drug Administration (FDA) 510(k) exemption registration for Bio Leg™; its robotic prosthetic knee. This registration marks a significant milestone in the company’s commitment to deliver its robotic prosthetic knee to the USA market in 2024 with Japanese-proven technologies. “Having…
Read MoreMarrow Access Technologies Partners with Spartan Medical to Provide Novel Cartilage Repair Therapy to US Veterans and Department of Defense Service Members
Marrow Access Technologies announced today that it signed a distribution agreement with Spartan Medical. The distribution agreement will provide patients in the Department of Veterans Affairs (VA) and Department of Defense (DoD) access to the SmartShot® Marrow Access Device, a novel solution for using the body’s own stem cells and healing capabilities to treat orthopedic…
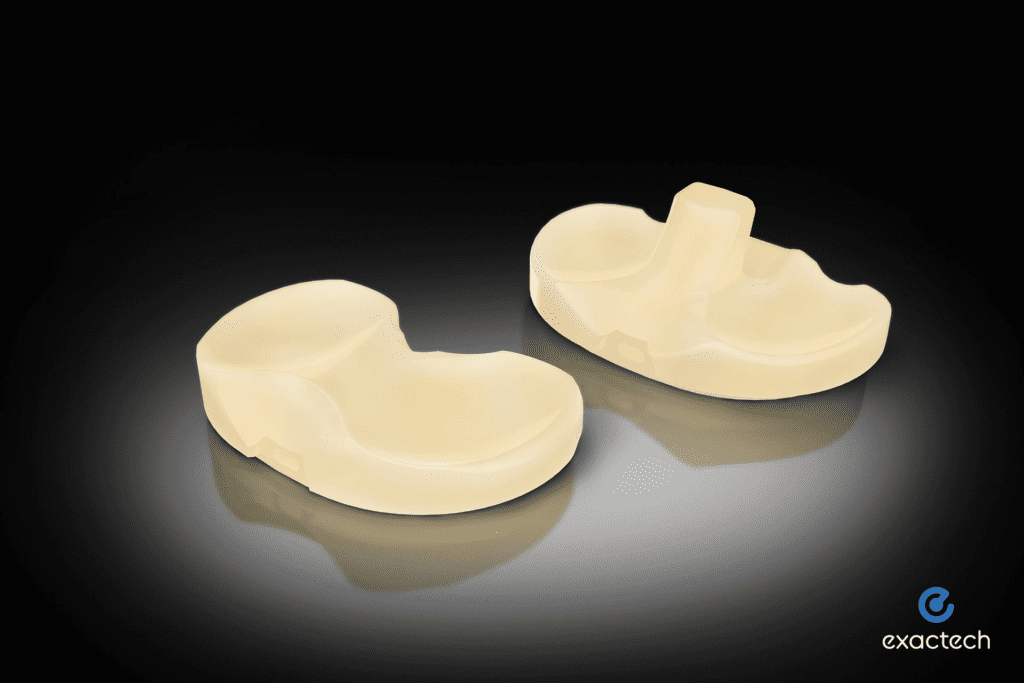
Read MoreExactech Announces FDA 510(k) Clearance for Advanced Activit-E™ Knee Replacement Polyethylene
Exactech, a developer and producer of innovative implants, instrumentation, and smart technologies for joint replacement surgery, announced 510(k) clearance from the U.S. Food and Drug Administration for its new, advanced Activit-E™ polyethylene for the Truliant® knee replacement system. “After years of research and development in polyethylene, Activit-E represents a breakthrough achievement for Exactech,” said Adam…
Read MoreViseon Inc. Announces Commercial Rollout and Initial Clinical Use of the 4K MaxView® System for Advanced Digital Visualization During Minimally Invasive Spine Surgery
Viseon Inc. announced the US commercial rollout and initial clinical use of the First-of-its-Kind 4K Advanced Visualization System for Minimally Invasive Spine Surgery. The Viseon MaxView 4K System is an enabling, towerless, state-of-the-art, advanced visualization system involving no capital equipment expense and occupying no operating room footprint, most accommodating in an ASC setting. The 4K…
Read MoreWillowWood Launches Fiberglass Meta® Shock X
Building on the success of META® Shock X, WillowWood launches the Fiberglass META® Shock X. The revolutionary META® feet combine responsive energy return with balance, stability, and impact protection. All META® feet feature the industry’s first unibody platform, free of hardware for a minimal and lightweight, but durable design. Fiberglass META Shock® X features our…
Read More