Archive for February 2023
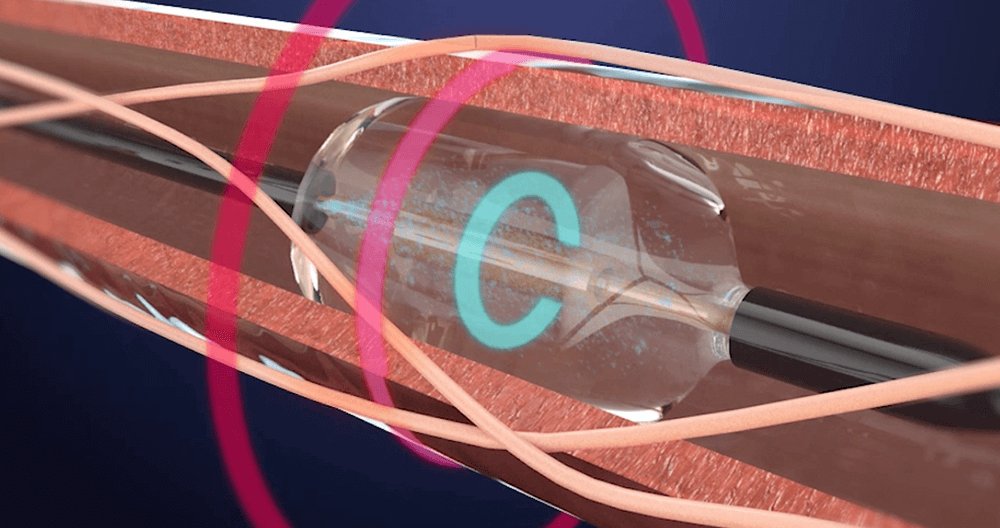
ReCor Medical Announces Two Concurrent JAMA Network Publications of Study Results on the Paradise Ultrasound Renal Denervation System for the Treatment of Hypertension
ReCor Medical, Inc. (“ReCor”) and its parent company, Otsuka Medical Devices Co., Ltd. (“Otsuka Medical Devices”) today announced that primary endpoint results from the RADIANCE II pivotal trial were published in the Journal of the American Medical Association (JAMA). Study results showed that the Paradise Ultrasound Renal Denervation (uRDN) System successfully reduced blood pressure compared to…
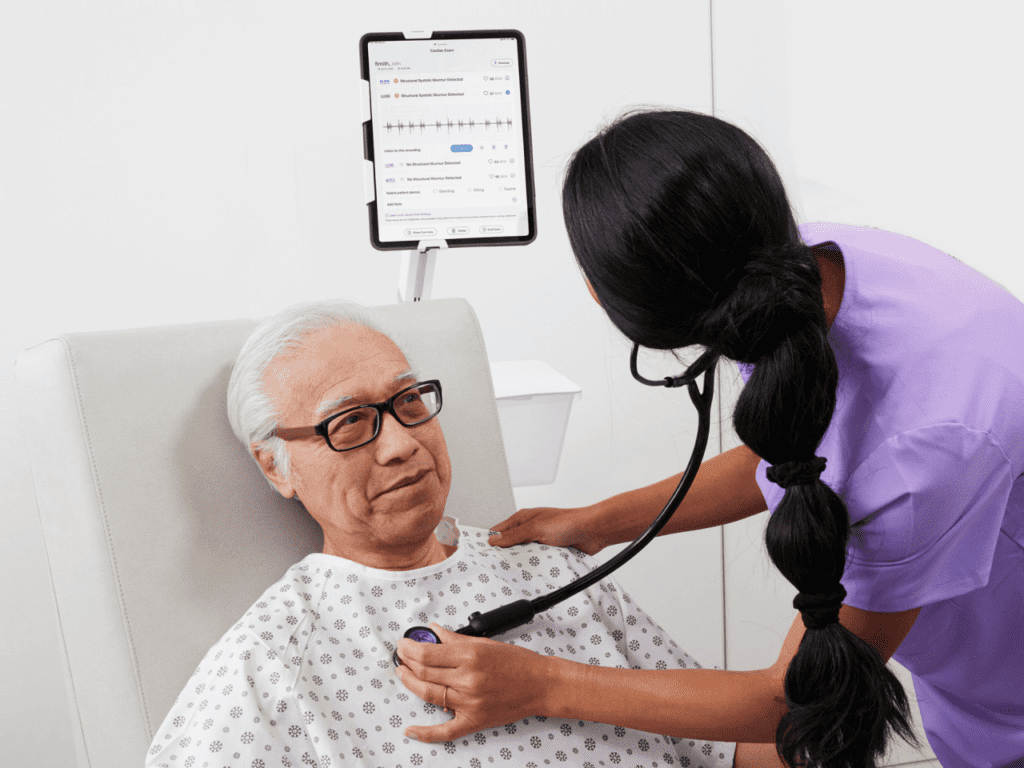
Read MoreEko Launches SENSORA™ Cardiac Disease Detection Platform
Eko, a digital health company applying artificial intelligence (AI) in the fight against heart and lung disease, today announced the launch of the SENSORA™ Cardiac Disease Detection Platform. SENSORA™ currently features AI that objectively identifies structural murmurs, a sign of valvular heart disease, and Care Pathway Analytics software that provides downstream visibility and metrics of the…
Read MoreCardio Diagnostics Holdings Inc to Debut PrecisionCHD™ at the American College of Cardiology’s 72nd Annual Scientific Session
Cardio Diagnostics Holdings Inc (Nasdaq: CDIO), an artificial intelligence-powered precision cardiovascular medicine company, today announced that its newest coronary heart disease test, PrecisionCHD™, will be presented at the American College of Cardiology’s 72nd Annual Scientific Session (ACC.23), taking place in New Orleans, LA, from March 4-6, 2023. PrecisionCHD, the company’s coronary heart disease early detection…
Read MoreLumendi Receives 510(k) Clearance for Two New Devices
Connecticut-based medical device innovator Lumendi, LLC announced that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for DiLumen EZ¹, a single-use, disposable endotherapy device for endoscopic mucosal resections (EMR) and difficult colonoscopies. The new, more cost-effective design, based on the long-standing success of the DiLumen EZ-Glide platform, has been modified and streamlined. “The…
Read MoreCurvaFix Launches Smaller-diameter CurvaFix® IM Implant
CurvaFix, Inc., a developer of medical devices to repair fractures in curved bones, announced the launch of its smaller-diameter, 7.5mm CurvaFix® IM Implant, designed to simplify surgery and provide strong, stable fixation in small-boned patients. The company will showcase the new 7.5mm intramedullary device, of which over two dozen have already been implanted in patients, at…
Read MoreTIOGA CARDIOVASCULAR, A SHIFAMED PORTFOLIO COMPANY, CLOSES $30M IN SERIES C FINANCING
Tioga Cardiovascular, a Shifamed portfolio company that aims to redefine structural heart valve replacement, announced today the first closing of its $30M Series C financing. Led by Cormorant Asset Management, with significant participation from The Capital Partnership, AMED Ventures, the PA MedTech VC Fund, and Shifamed angel investors, the financing will be used to continue…
Read MoreHyperfine, Inc. Announces FDA Clearance for Improved AI-Powered Software and Expanded Field of View for the Swoop® Portable MR Imaging® System
Hyperfine, Inc., the groundbreaking medical device company that created the Swoop® system, the world’s first FDA-cleared portable magnetic resonance imaging (MRI) device for imaging of the brain, today announced the U.S. Food and Drug Administration (FDA) 510(k) clearance and launch of the company’s upgraded AI-powered software. The most recently cleared Swoop® software improves the image…
Read MoreCatalyst Receives FDA 510(k) Clearance for its Convertible Stemmed Total Shoulder Arthroplasty System
Catalyst OrthoScience Inc. (Catalyst), a medical device company focused on the upper extremity orthopedics market, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its fully convertible stemmed total shoulder arthroplasty system with an ellipsoid anatomic head. This is the market’s first stemmed arthroplasty system featuring an ellipsoid humeral head,…
Read MoreGlobus Medical and NuVasive to Combine in All-Stock Transaction to Create Innovative Global Musculoskeletal Company Focused on Patient Care
Globus Medical (NYSE: GMED), a leading musculoskeletal solutions company, and NUVASIVE (NASDAQ: NUVA), the leader in spine technology innovation, today announced they have entered into a definitive agreement to combine in an all-stock transaction. The transaction brings together two well-regarded technology companies in the musculoskeletal industry, which have a shared vision focused on innovation in…
Read MoreCoolHealth Enters U.S. Market With TargetCool™, A Breakthrough FDA-Class II Medical Device Offering Unprecedented Precise, Rapid Cooling For Pain Management And Bruise Minimization
Recens, Inc. dba CoolHealth (“CoolHealth”), a venture-backed medical device company developing innovative cooling technologies, is entering the U.S. market with a breakthrough FDA-Class II medical device, TargetCool™. Leveraging groundbreaking, tuneable cooling technology, TargetCool rapidly cools the skin allowing providers to specifically set a “target” skin temperature down to the exact degree and second– a first in medical…
Read More